IAS 2017 IASconference Conflict of Interest No conflicts










- Slides: 23

#IAS 2017 | @IAS_conference Conflict of Interest “No conflicts of interest to declare”

#IAS 2017 | @IAS_conference Track B Rapporteurs Denise Hsu Lara Coelho Vincent Marconi Paula Munderi

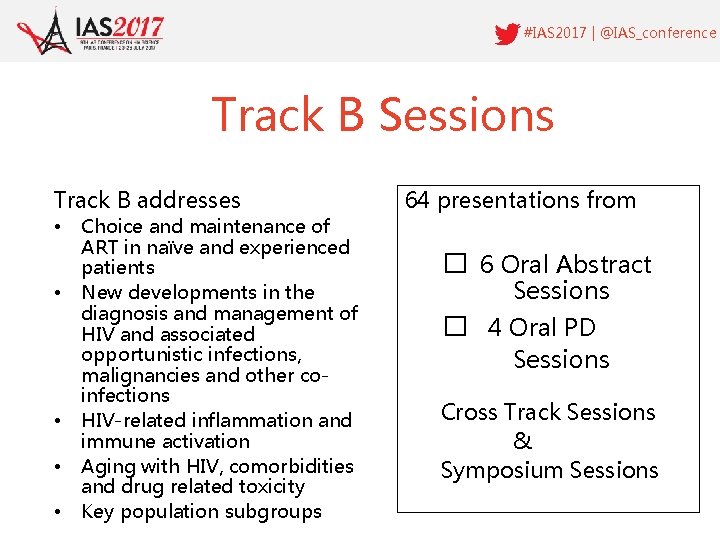
#IAS 2017 | @IAS_conference Track B Sessions Track B addresses • • • Choice and maintenance of ART in naïve and experienced patients New developments in the diagnosis and management of HIV and associated opportunistic infections, malignancies and other coinfections HIV-related inflammation and immune activation Aging with HIV, comorbidities and drug related toxicity Key population subgroups 64 presentations from � 6 Oral Abstract Sessions � 4 Oral PD Sessions Cross Track Sessions & Symposium Sessions

#IAS 2017 | @IAS_conference New data from Phase 3 Studies FIXED DOSE COMBINATIONS FOR TREATMENT NAÏVE ADULTS

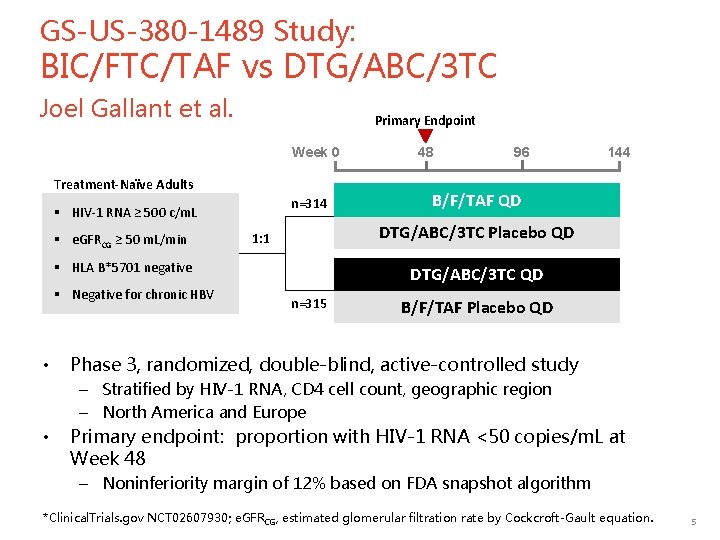
GS-US-380 -1489 Study: BIC/FTC/TAF vs DTG/ABC/3 TC Joel Gallant et al. Primary Endpoint Week 0 Treatment-Naïve Adults n=314 § HIV-1 RNA ≥ 500 c/m. L § e. GFRCG ≥ 50 m. L/min • 96 144 B/F/TAF QD DTG/ABC/3 TC Placebo QD 1: 1 § HLA B*5701 negative § Negative for chronic HBV 48 DTG/ABC/3 TC QD n=315 B/F/TAF Placebo QD Phase 3, randomized, double-blind, active-controlled study – Stratified by HIV-1 RNA, CD 4 cell count, geographic region – North America and Europe • Primary endpoint: proportion with HIV-1 RNA <50 copies/m. L at Week 48 – Noninferiority margin of 12% based on FDA snapshot algorithm *Clinical. Trials. gov NCT 02607930; e. GFRCG, estimated glomerular filtration rate by Cockcroft-Gault equation. 5

GS-US-380 -1489 Study: Virologic outcome at 48 weeks % Treatment Difference (95% CI) Virologic Outcome HIV-1 RNA <50 c/m. L, % 100 92. 4 93. 0 80 Favors DTG/ABC/3 TC (n=315) Favors B/F/TAF 60 -0. 6 HIV-1 RNA < 50 copies/m. L 40 20 0 1. 0 HIV-1 RNA < 50 copies/m. L 290 314 • B/F/TAF (n=314) 293 315 2. 5 HIV-1 RNA ≥ 50 copies/m. L 3 314 8 315 6. 7 3. 6 4. 4 No Virologic Data 21 314 -4. 8 -12 -8 -4 0 4 8 12 14 315 Non-inferiority confirmed by pre-specified analyses for HIV-1 RNA < 50 copies/m. L: – Per protocol: B/F/TAF 99. 3% vs DTG/ABC/3 TC 98. 6% (p=0. 43) – Missing=Failure: B/F/TAF 92. 4% vs DTG/ABC/3 TC 93. 3% (p=0. 65) – Missing=Excluded: B/F/TAF 99. 3% vs DTG/ABC/3 TC 97. 7% (p=0. 10) • Mean CD 4 increase from baseline at Week 48: – B/F/TAF +233 cells/µL vs DTG/ABC/3 TC +229 cells/µL (p=0. 81) 6

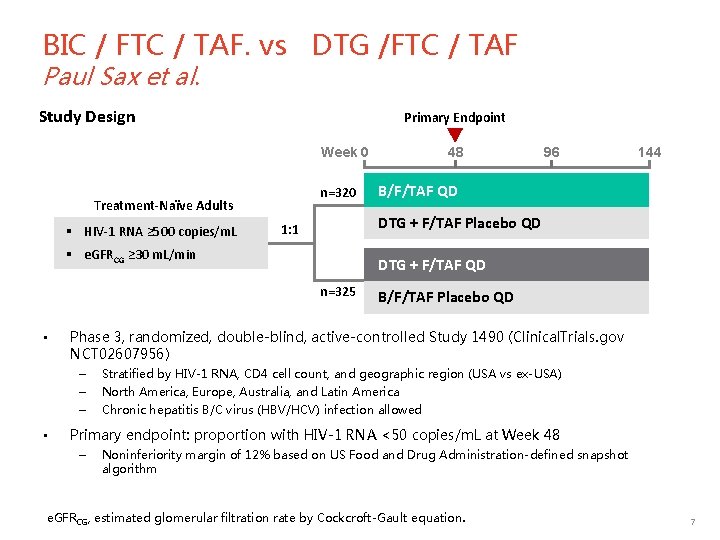
BIC / FTC / TAF. vs DTG /FTC / TAF Paul Sax et al. Study Design Primary Endpoint 48 Week 0 n=320 Treatment-Naïve Adults § HIV-1 RNA ≥ 500 copies/m. L B/F/TAF QD DTG + F/TAF QD n=325 B/F/TAF Placebo QD Phase 3, randomized, double-blind, active-controlled Study 1490 (Clinical. Trials. gov NCT 02607956) – – – • 144 DTG + F/TAF Placebo QD 1: 1 § e. GFRCG ≥ 30 m. L/min • 96 Stratified by HIV-1 RNA, CD 4 cell count, and geographic region (USA vs ex-USA) North America, Europe, Australia, and Latin America Chronic hepatitis B/C virus (HBV/HCV) infection allowed Primary endpoint: proportion with HIV-1 RNA <50 copies/m. L at Week 48 – Noninferiority margin of 12% based on US Food and Drug Administration-defined snapshot algorithm e. GFRCG, estimated glomerular filtration rate by Cockcroft-Gault equation. 7

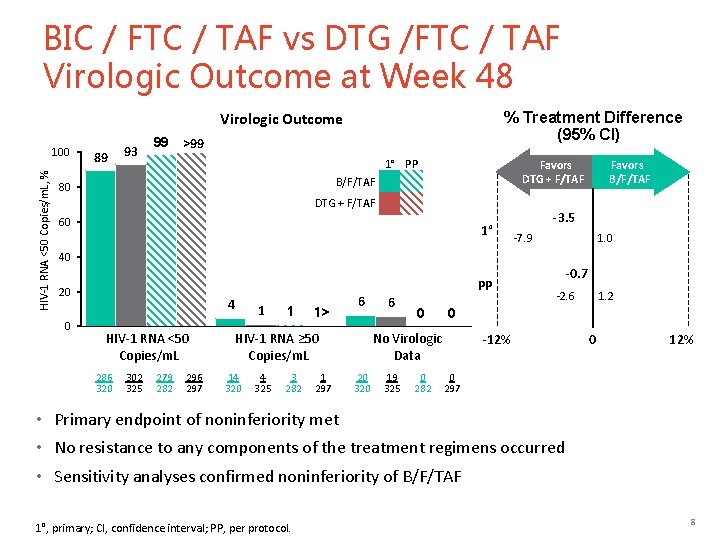
BIC / FTC / TAF vs DTG /FTC / TAF Virologic Outcome at Week 48 % Treatment Difference (95% CI) Virologic Outcome HIV-1 RNA <50 Copies/m. L, % 100 89 93 99 >99 1° PP Favors DTG + F/TAF B/F/TAF 80 DTG + F/TAF 60 1° Favors B/F/TAF -3. 5 -7. 9 1. 0 40 20 0 4 HIV-1 RNA <50 Copies/m. L 286 320 302 325 279 282 296 297 1 1 1> 6 HIV-1 RNA ≥ 50 Copies/m. L 14 320 4 325 3 282 1 297 PP 6 0 19 325 0 282 -2. 6 1. 2 0 No Virologic Data 20 320 -0. 7 -12% 0 297 • Primary endpoint of noninferiority met • No resistance to any components of the treatment regimens occurred • Sensitivity analyses confirmed noninferiority of B/F/TAF 1°, primary; CI, confidence interval; PP, per protocol. 8

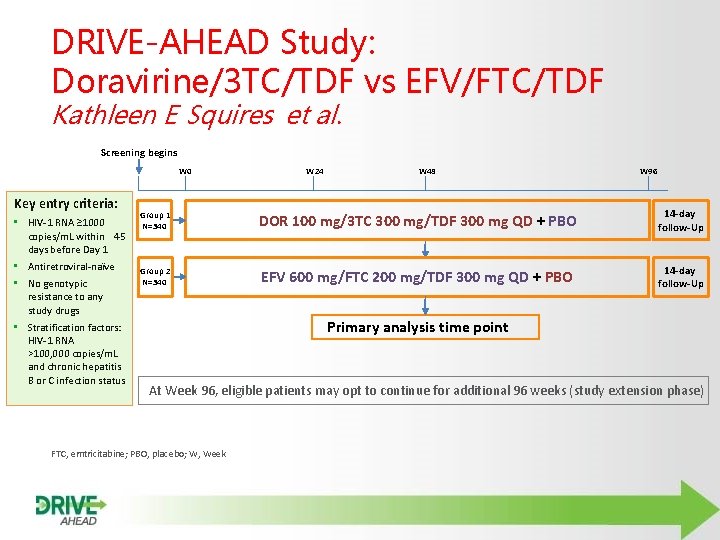
DRIVE-AHEAD Study: Doravirine/3 TC/TDF vs EFV/FTC/TDF Kathleen E Squires et al. Screening begins W 0 Key entry criteria: • HIV-1 RNA ≥ 1000 copies/m. L within 45 days before Day 1 • Antiretroviral-naїve • No genotypic resistance to any study drugs • Stratification factors: HIV-1 RNA >100, 000 copies/m. L and chronic hepatitis B or C infection status W 24 W 48 W 96 Group 1 N=340 DOR 100 mg/3 TC 300 mg/TDF 300 mg QD + PBO 14 -day follow-Up Group 2 N=340 EFV 600 mg/FTC 200 mg/TDF 300 mg QD + PBO 14 -day follow-Up Primary analysis time point At Week 96, eligible patients may opt to continue for additional 96 weeks (study extension phase) FTC, emtricitabine; PBO, placebo; W, Week

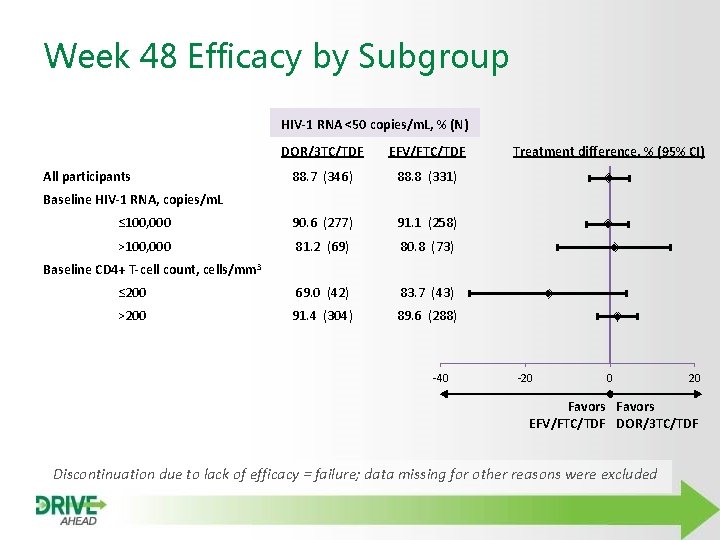
Week 48 Efficacy by Subgroup HIV-1 RNA <50 copies/m. L, % (N) DOR/3 TC/TDF EFV/FTC/TDF 88. 7 (346) 88. 8 (331) ≤ 100, 000 90. 6 (277) 91. 1 (258) >100, 000 81. 2 (69) 80. 8 (73) ≤ 200 69. 0 (42) 83. 7 (43) >200 91. 4 (304) 89. 6 (288) All participants Treatment difference, % (95% CI) Baseline HIV-1 RNA, copies/m. L Baseline CD 4+ T-cell count, cells/mm 3 -40 -20 0 20 Favors EFV/FTC/TDF DOR/3 TC/TDF Discontinuation due to lack of efficacy = failure; data missing for other reasons were excluded

#IAS 2017 | @IAS_conference New data SWITCH STRATEGIES AND DRUG OPTIMISATION

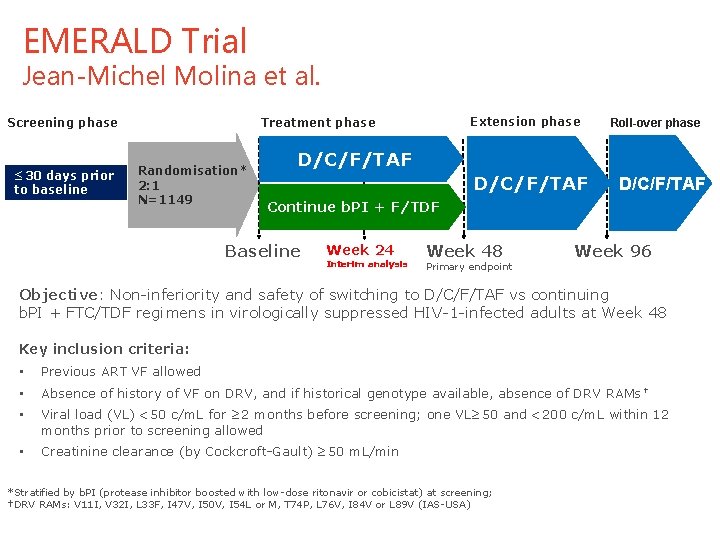
EMERALD Trial Jean-Michel Molina et al. Screening phase ≤ 30 days prior to baseline Extension phase Treatment phase Randomisation* 2: 1 N=1149 Roll-over phase D/C/F/TAF Continue b. PI + F/TDF Baseline Week 24 Interim analysis Week 48 Primary endpoint Week 96 Objective: Non-inferiority and safety of switching to D/C/F/TAF vs continuing b. PI + FTC/TDF regimens in virologically suppressed HIV-1 -infected adults at Week 48 Key inclusion criteria: • Previous ART VF allowed • Absence of history of VF on DRV, and if historical genotype available, absence of DRV RAMs † • Viral load (VL) <50 c/m. L for ≥ 2 months before screening; one VL≥ 50 and <200 c/m. L within 12 months prior to screening allowed • Creatinine clearance (by Cockcroft-Gault) ≥ 50 m. L/min *Stratified by b. PI (protease inhibitor boosted with low-dose ritonavir or cobicistat) at screening; †DRV RAMs: V 11 I, V 32 I, L 33 F, I 47 V, I 50 V, I 54 L or M, T 74 P, L 76 V, I 84 V or L 89 V (IAS-USA) 12

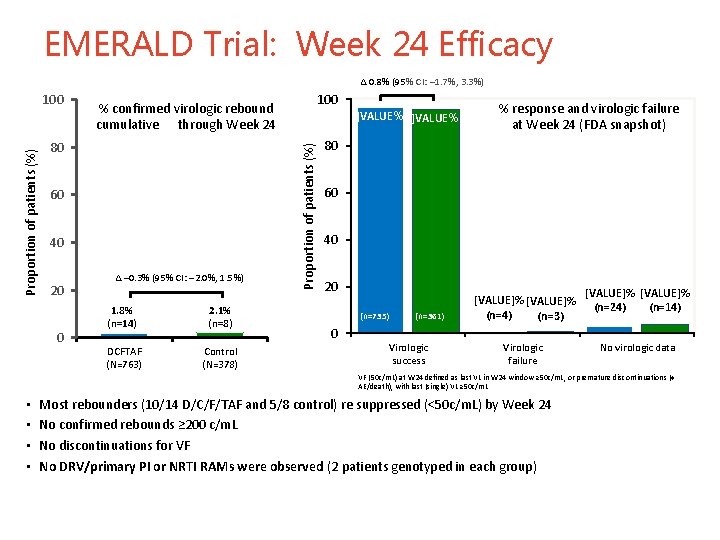
EMERALD Trial: Week 24 Efficacy ∆ 0. 8% (95% CI: – 1. 7%, 3. 3%) 80 60 40 20 0 100 % confirmed virologic rebound cumulative through Week 24 ∆ – 0. 3% (95% CI: – 2. 0%, 1. 5%) 1. 8% (n=14) 2. 1% (n=8) DCFTAF (N=763) Control (N=378) ]VALUE%[ Proportion of patients (%) 100 % response and virologic failure at Week 24 (FDA snapshot) 80 60 40 20 (n=735) 0 (n=361) Virologic success [VALUE]% (n=4) (n=3) Virologic failure [VALUE]% (n=14) (n=24) No virologic data VF (50 c/m. L) at W 24 defined as last VL in W 24 window ≥ 50 c/m. L, or premature discontinuations (≠ AE/death), with last (single) VL ≥ 50 c/m. L • • Most rebounders (10/14 D/C/F/TAF and 5/8 control) re suppressed (<50 c/m. L) by Week 24 No confirmed rebounds ≥ 200 c/m. L No discontinuations for VF No DRV/primary PI or NRTI RAMs were observed (2 patients genotyped in each group) 1 3

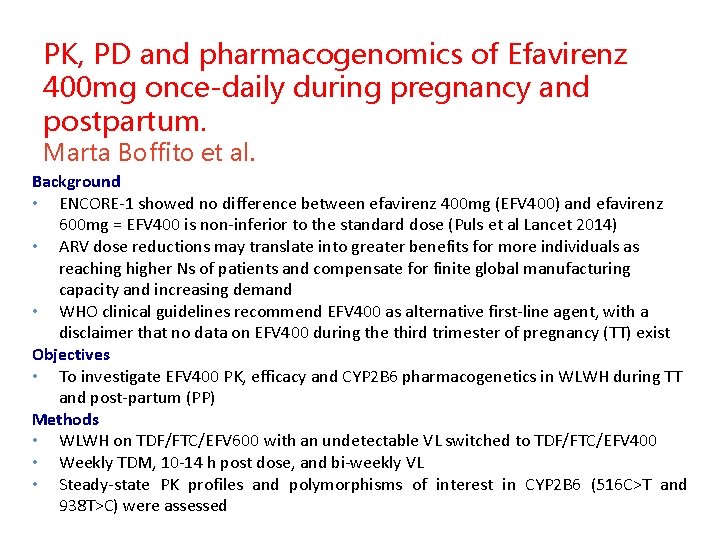
PK, PD and pharmacogenomics of Efavirenz 400 mg once-daily during pregnancy and postpartum. Marta Boffito et al. Background • ENCORE-1 showed no difference between efavirenz 400 mg (EFV 400) and efavirenz 600 mg = EFV 400 is non-inferior to the standard dose (Puls et al Lancet 2014) • ARV dose reductions may translate into greater benefits for more individuals as reaching higher Ns of patients and compensate for finite global manufacturing capacity and increasing demand • WHO clinical guidelines recommend EFV 400 as alternative first-line agent, with a disclaimer that no data on EFV 400 during the third trimester of pregnancy (TT) exist Objectives • To investigate EFV 400 PK, efficacy and CYP 2 B 6 pharmacogenetics in WLWH during TT and post-partum (PP) Methods • WLWH on TDF/FTC/EFV 600 with an undetectable VL switched to TDF/FTC/EFV 400 • Weekly TDM, 10 -14 h post dose, and bi-weekly VL • Steady-state PK profiles and polymorphisms of interest in CYP 2 B 6 (516 C>T and 938 T>C) were assessed

Results: PK parameters and profiles Cmax, Ctrough and AUC in TT were 7%, 27% and 16% lower compared to PP but within ranges of those measured for EFV 600 during TT by Schalkwijk et al. (2016) and those measured in ARVnaïve patients on EFV 400 in ENCORE-1 (Dickinson et al. 2015)

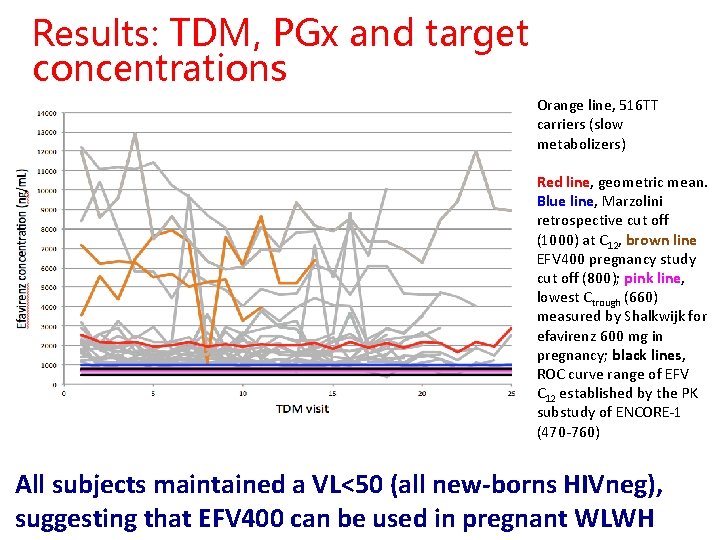
Results: TDM, PGx and target concentrations Orange line, 516 TT carriers (slow metabolizers) Red line, geometric mean. Blue line, Marzolini retrospective cut off (1000) at C 12, brown line EFV 400 pregnancy study cut off (800); pink line, lowest Ctrough (660) measured by Shalkwijk for efavirenz 600 mg in pregnancy; black lines, ROC curve range of EFV C 12 established by the PK substudy of ENCORE-1 (470 -760) All subjects maintained a VL<50 (all new-borns HIVneg), suggesting that EFV 400 can be used in pregnant WLWH

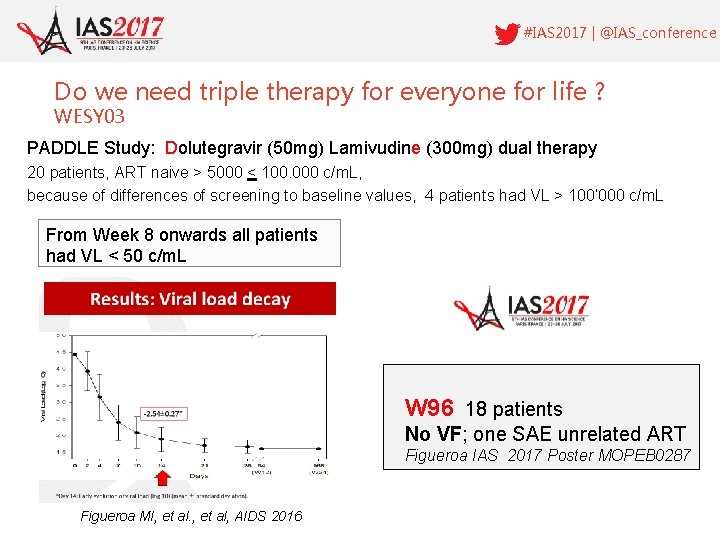
#IAS 2017 | @IAS_conference Do we need triple therapy for everyone for life ? WESY 03 PADDLE Study: Dolutegravir (50 mg) Lamivudine (300 mg) dual therapy 20 patients, ART naive > 5000 < 100. 000 c/m. L, because of differences of screening to baseline values, 4 patients had VL > 100‘ 000 c/m. L From Week 8 onwards all patients had VL < 50 c/m. L W 96 18 patients No VF; one SAE unrelated ART Figueroa IAS 2017 Poster MOPEB 0287 Figueroa MI, et al, AIDS 2016

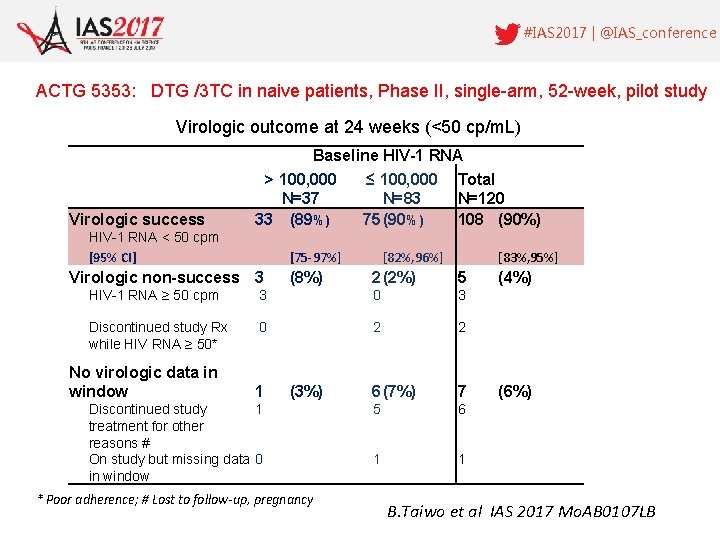
#IAS 2017 | @IAS_conference ACTG 5353: DTG /3 TC in naive patients, Phase II, single-arm, 52 -week, pilot study Virologic outcome at 24 weeks (<50 cp/m. L) Virologic success Baseline HIV-1 RNA > 100, 000 ≤ 100, 000 Total N=37 N=83 N=120 33 (89%) 75 (90%) 108 (90%) HIV-1 RNA < 50 cpm [95% CI] [75 -97%] Virologic non-success 3 [82%, 96%] [83%, 95%] (8%) 2 (2%) 5 (4%) HIV-1 RNA ≥ 50 cpm 3 0 3 Discontinued study Rx while HIV RNA ≥ 50* 0 2 2 1 (3%) 6 (7%) 7 (6%) 5 6 1 1 No virologic data in window Discontinued study 1 treatment for other reasons # On study but missing data 0 in window * Poor adherence; # Lost to follow-up, pregnancy B. Taiwo et al IAS 2017 Mo. AB 0107 LB

HCV – HIV coinfection #IAS 2017 | @IAS_Conference

The EXPEDITION-2 Study Karine Lacombe et al. Efficacy and Safety of Glecaprevir/Pibrentasvir in Patients Co-infected with Hepatitis C Virus and HIV-1 Study design Efficacy 98% overall SVR 12 with no relapses in patients without or with cirrhosis following 8 or 12 weeks of G/P respectively 99% SVR 12 rate with no virologic failures with 8 -week G/P in HCV/HIV-1 co-infected patients without cirrhosis Safety: G/P was well tolerated both in cirrhotic and non cirrhotic patients. The G/P regimen could be the first 8 -week, pangenotypic treatment option for HCV/HIV-1 coinfected patients without cirrhosis

#IAS 2017 | @IAS_conference HIV Drug Resistance Surveillance Pre-Treatment Drug Resistance (PDR) is increasing worldwide especially in eastern and southern Africa • NNRTI DRM in adults > 10% in Uganda, Namibia, Zimbabwe, Nicaragua, Guatemala, Argentina in adults • PDR in infants below 18 months 5055% Multicountry surveys incl. Mozambique, Swaziland, Uganda, Zimbabwe, South Africa

Conclusion • ART regimens that are simpler more efficacious and better tolerated • Promising new treatment for HCV coinfection • Sobering data from global HVDR surveillance

Thank You #IAS 2017 | @IAS_Conference
 Conflict of interest in business
Conflict of interest in business Effective annual rate
Effective annual rate Compound interest meaning
Compound interest meaning Real vs nominal interest rate
Real vs nominal interest rate Conflict of interest penelitian adalah
Conflict of interest penelitian adalah El 312 usps
El 312 usps Chapter 4 business ethics and social responsibility
Chapter 4 business ethics and social responsibility Google scholar
Google scholar Conflict with interest
Conflict with interest You love going to soccer practice every friday night
You love going to soccer practice every friday night What is conflict and conflict resolution?
What is conflict and conflict resolution? The definition of external conflict
The definition of external conflict Conflict definition literature
Conflict definition literature Why do territorial conflicts arise among religious groups
Why do territorial conflicts arise among religious groups Motivational conflict marketing
Motivational conflict marketing Fahrenheit 451 internal conflict
Fahrenheit 451 internal conflict How do you think wolf packs form on expressways
How do you think wolf packs form on expressways What is the rising action in freak the mighty
What is the rising action in freak the mighty Conflict of animal farm
Conflict of animal farm Chapter 9 resolving conflicts and preventing violence
Chapter 9 resolving conflicts and preventing violence External conflict definiton
External conflict definiton The lady or the tiger climax
The lady or the tiger climax Cuda shared memory size
Cuda shared memory size Is to kill a mockingbird a bildungsroman
Is to kill a mockingbird a bildungsroman