HUMAN IMMUNODEFICIENCY VIRUS INFECTION Acquired immunodeficiency syndrome was












- Slides: 23

HUMAN IMMUNODEFICIENCY VIRUS INFECTION

Acquired immunodeficiency syndrome was first reported in 1981 in the Morbidity and Mortality Weekly Report under the title “ Pneumocystis pneumonia – Los Angeles”. In 20 years, the AIDS epidemic has grown from a series of small outbreaks in several risk groups throughout the United States and western Europe into a global public health calamity. Tremendous advances have been made in understanding the molecular mechanisms, in achieving of antiretroviral therapy and blood-supply safety. Although the disease was first encountered in homosexual men andinjection-drug users, the risk groups soon included transfusion recipients, infants, female sexual contact of infected men, prisoners, Haitians and Africans.

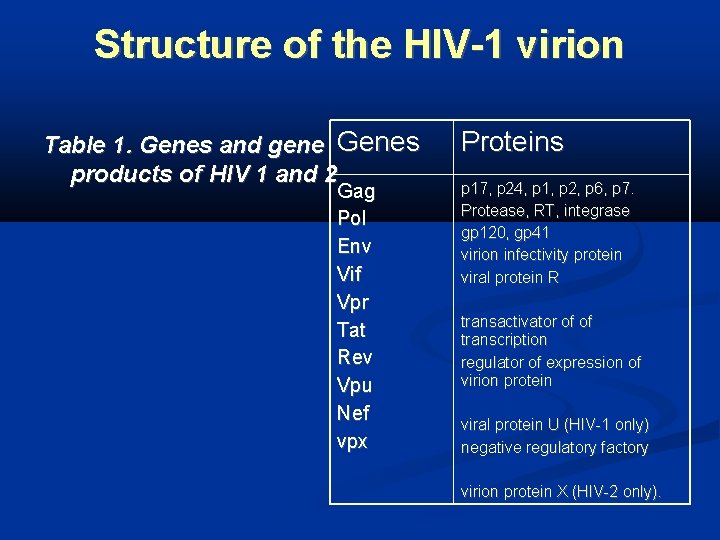
Structure of the HIV-1 virion Table 1. Genes and gene Genes products of HIV 1 and 2 Gag Pol Env Vif Vpr Tat Rev Vpu Nef vpx Proteins p 17, p 24, p 1, p 2, p 6, p 7. Protease, RT, integrase gp 120, gp 41 virion infectivity protein viral protein R transactivator of of transcription regulator of expression of virion protein viral protein U (HIV-1 only) negative regulatory factory virion protein X (HIV-2 only).

The life cycle of HIV-1 The major steps in the HIV life are: Afferent functions 1. Binding and entry. 2. Reverse transcription, nuclear import, and integration of viral DNA Efferent functions • Viral transcription (production of m. RNA • Production of viral regulatory factors • Virion assembly. • Virus budding and release

Pathogenesis of HIV disease The main target of HIV infection is the CD 4 -positive-lymphocyte population, although infection of tymocytes, macrophages and dendritic cells, can occur. Additional co-receptor which are used for virus entry into CD 4 -T cells are: 1. CC-chemokine receptor CCR 5 2. CXC-chemokine receptor (CXCR 4)-.

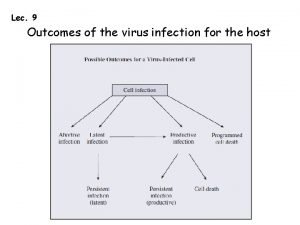
Acute HIV infection Viral load levels in the blood rise; Dissemination of the virions CD 4 T-cells count decrease Appearance of HIV-specific CTL (cytotoxic T lymphocytes) which kill infected cells and secrete antiviral cytokines and chemokines). Chronic HIV infection CD 4 T-cells number and function continue to decline during the period of clinical latency; Defects in T-cell function/loss of specific responses to recall antigens; Abnormalities in B-cell, natural killer, monocytes and dendritic cell functions Altered cytokine secretion with reduced production of IFN-gamma, IL -2 and IL-12 may occur The virus continue to replicate at all stages of infection

Mechanisms of immune depletion are: Direct cytopathic effect Syncytium or multinucleated cell formation, Apoptosis (programmed cell death). Removal of infected CD 4 T cells and dendritic cells by the vigorous HIVspecific CTL responses

Clinical manifestations Classification of HIV-1 disease into six stages is based on a combination of clinical features and CD 4 count: 1. Initial infection (acute seroconversion syndrome) 2. Early HIV-1 disease 3. Intermediate HIV-1 disease 4. Late HIV-1 disease 5. Advanced HIV-1 disease . 6. Terminal HIV-1 disease

Initial infection (acute seroconversion syndrome) – usually occurs within 2 to 6 weeks (median=21 days) after exposure to the virus. - may be asymptomatic or - may evolve with symptoms of flu-like or mononucleosis-like illness The most common symptoms are: • fever, • lymphadenopathy • pharyngitis, esophagitis, aphthous ulcerations • myalgias, arthralgias • headache • diarrhea • morbilliform skin eruption • neurologic manifestations: meningitis, peripheral neuropathy, • myopathy, cranial nerve palsies.

Early HIV-1 disease - is defined by a CD 4 cell count greater than 500 cells/mmc. Most persons are asymptomatic. Among symptoms: -lymphadenopathy (cervical, axillary and inguinal chains), - dermatologic abnormalities: seboreic dermatitis, perifolliculitis, eosinophilic folliculitis, - oral lesions: aphtous ulcerations, hairy leucoplakia. Intermediate stage of HIV-1 disease – is defined by a CD 4 count between 200 and 500 cells/mmc and could evolves without symptoms or with mild disease manifestations: - Recurrent herpes simplex infection - Oropharyngeal/vaginal candidiasis - Varicella zoster virus infection - Weight loss - Bacterial sinusitis, bronchitis, pneumonia - Headache, myalgias, arthralgias

Late-stage disease – is defined by a CD 4 cell count between 50 and 200 cells/mmc. These patients have a great risk of developing : • opportunistic infections such as Pneumocystis carini pneumonia, Toxoplasma gondii infection, cryptosporidiosis, tuberculosis, Kaposi’s sarcoma, esophagial candidiasis and • hematologic abnormalities (anemia, neutropenia, idiopathic thrombocytopenia). Advanced HIV disease – is defined as a CD 4 count of less than 50 cells/mmc. The most frequent conditions seen during this stage are: MAC disease, cryptococcal meningitis, cytomegalovirus retinitis, progressive multifocal leukoencephalopathy. Terminal HIV disease – symptoms of disease cannot be controlled because no treatment are available The management includes psychological support, family support, and pain management.

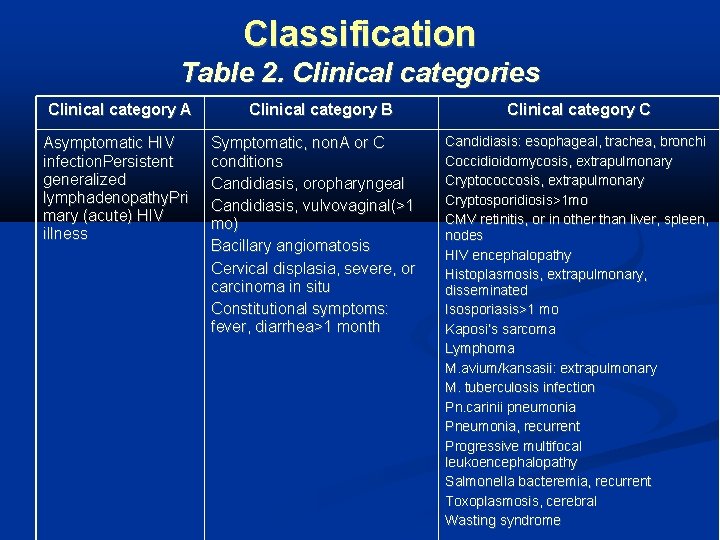
Classification Table 2. Clinical categories Clinical category A Asymptomatic HIV infection. Persistent generalized lymphadenopathy. Pri mary (acute) HIV illness Clinical category B Symptomatic, non. A or C conditions Candidiasis, oropharyngeal Candidiasis, vulvovaginal(>1 mo) Bacillary angiomatosis Cervical displasia, severe, or carcinoma in situ Constitutional symptoms: fever, diarrhea>1 month Clinical category C Candidiasis: esophageal, trachea, bronchi Coccidioidomycosis, extrapulmonary Cryptococcosis, extrapulmonary Cryptosporidiosis>1 mo CMV retinitis, or in other than liver, spleen, nodes HIV encephalopathy Histoplasmosis, extrapulmonary, disseminated Isosporiasis>1 mo Kaposi’s sarcoma Lymphoma M. avium/kansasii: extrapulmonary M. tuberculosis infection Pn. carinii pneumonia Pneumonia, recurrent Progressive multifocal leukoencephalopathy Salmonella bacteremia, recurrent Toxoplasmosis, cerebral Wasting syndrome

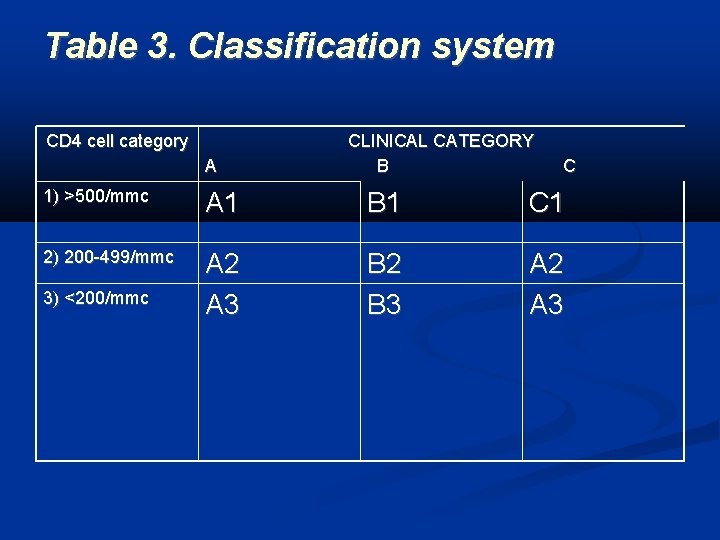
Table 3. Classification system CD 4 cell category A CLINICAL CATEGORY B C 1) >500/mmc A 1 B 1 C 1 2) 200 -499/mmc A 2 A 3 B 2 B 3 A 2 A 3 3) <200/mmc

Pulmonary diseases Any CD 4 level: CD 4<200/mmc CD 4<100/mmc - Cryptococcosis - Coccidioidomycosis - Histoplasmosis CD 4<50/mmc - Aspergilosis - M. avium

Neurologic manifestations Brain involvement A. Focal lesions B. Nonfocal complications Spinal cord Meninges Peripheral nerve and root lymphomatous meningitis;

Table 4. Classification of oral lesions: Viral lesions Neoplastic lesions Hairy leukoplakia Herpes simplex Herpes zoster Cytomegalovirus • Kaposi’s sarcoma • Non-Hodgkin’s lymphoma • Hodgkin’s lymphoma Fungal lesions Candidiasis Histoplasmosis Cryptococcosis Autoimmune/idiopathic lesions • Salivary gland disease • Aphtous ulcers • Abnormal pigmentation Bacterial lesions Periodontal disease Necrotizing stomatitis Mycobacterium avium complex Bacillary angiomatosis

Dermatologic complications Table 5. Skin lesions Maculopapular lesions Moluscum contagiosum Syphilis Mycobacterial infection. Kaposi’s sarcoma Nodular, verrucous, and/or ulcerative lesions Bacillary angiomatosis Cryptococcosis Sporotrichosis MAC infections Kaposi’s sarcoma Vesicular, bulous or pustular lesions Herpes simplex virus Varicella-zoster Cytomegalovirus Staphylococcal impetigo Stevens-Johnson syndrome Papulosquamous lesions Seborrheic dermatitis Dry-skin syndrome Psoriasis Norwegian scabies Staphylococcal folliculitis Eosinophilicfolliculitis

Table 6. Acute infectious diarrhea • Campylobacter jejuni • Clostridium difficile • Enteric viruses • Enteroadherent E. coli • Salmonella • Shigella • Cryptosporidia • Cytomegalovirus • Entamoeba histolytica • Isospora belli • Microsporidia • Mycobacterium avium

Spectrum of associated malignancies Kaposi’s sarcoma Primary CNS lymphoma Non-Hodgkin’s lymphoma Cervical carcinoma Hodgkin’s disease Seminoma

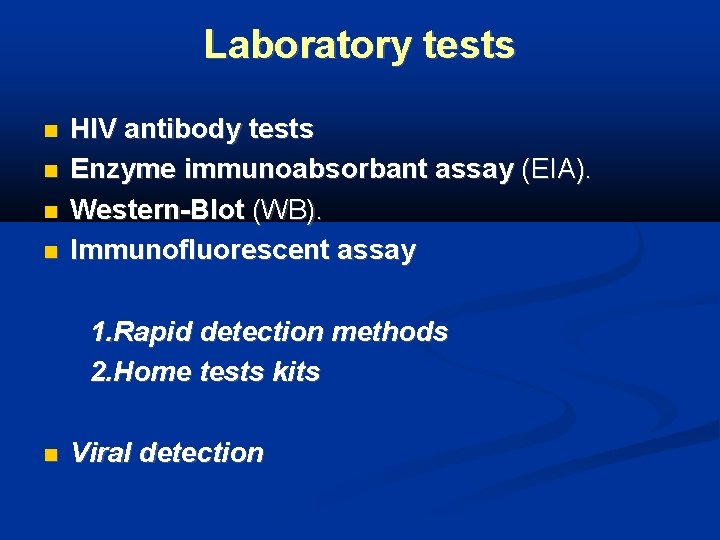
Laboratory tests HIV antibody tests Enzyme immunoabsorbant assay (EIA). Western-Blot (WB). Immunofluorescent assay 1. Rapid detection methods 2. Home tests kits Viral detection

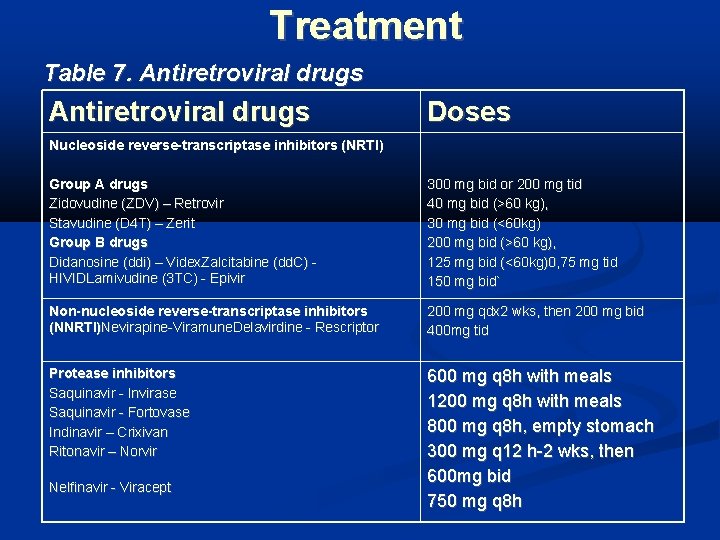
Treatment Table 7. Antiretroviral drugs Doses Nucleoside reverse-transcriptase inhibitors (NRTI) Group A drugs Zidovudine (ZDV) – Retrovir Stavudine (D 4 T) – Zerit Group B drugs Didanosine (ddi) – Videx. Zalcitabine (dd. C) HIVIDLamivudine (3 TC) - Epivir 300 mg bid or 200 mg tid 40 mg bid (>60 kg), 30 mg bid (<60 kg) 200 mg bid (>60 kg), 125 mg bid (<60 kg)0, 75 mg tid 150 mg bid` Non-nucleoside reverse-transcriptase inhibitors (NNRTI)Nevirapine-Viramune. Delavirdine - Rescriptor 200 mg qdx 2 wks, then 200 mg bid 400 mg tid Protease inhibitors Saquinavir - Invirase Saquinavir - Fortovase Indinavir – Crixivan Ritonavir – Norvir 600 mg q 8 h with meals 1200 mg q 8 h with meals 800 mg q 8 h, empty stomach 300 mg q 12 h-2 wks, then 600 mg bid 750 mg q 8 h Nelfinavir - Viracept

Initial regimens Preferred: - 2 nucleosides and a PI - 2 nucleosides and a NNRTI Under evaluation: 3 nucleosides In patients with CD 4<50/mmc or viral load >100 000 c/ml: - 2 NRTIs + 2 PI - 2 NRTIs + PI + NNRTI HAART (highly active antiretroviral therapy) is an antiretroviral regimen that can be expected to reduce the viral load to<50 c/ml in treatment-naïve patients.

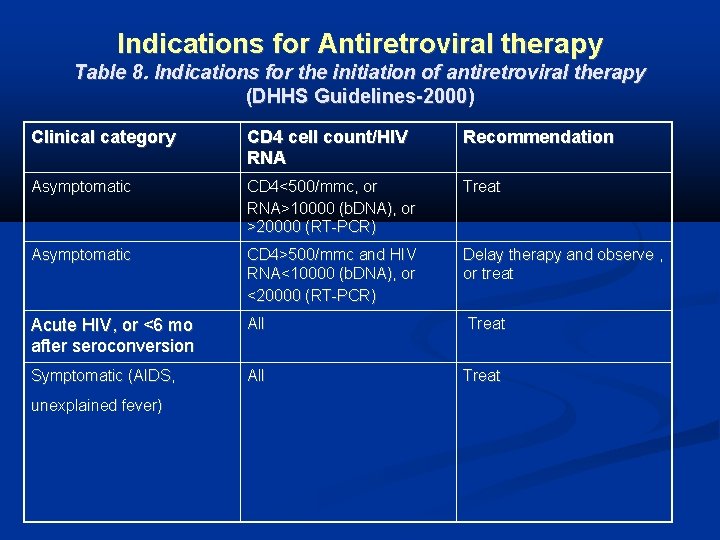
Indications for Antiretroviral therapy Table 8. Indications for the initiation of antiretroviral therapy (DHHS Guidelines-2000) Clinical category CD 4 cell count/HIV RNA Recommendation Asymptomatic CD 4<500/mmc, or RNA>10000 (b. DNA), or >20000 (RT-PCR) Treat Asymptomatic CD 4>500/mmc and HIV RNA<10000 (b. DNA), or <20000 (RT-PCR) Delay therapy and observe , or treat Acute HIV, or <6 mo after seroconversion All Treat Symptomatic (AIDS, All Treat unexplained fever)
 Monocyte derived dendritic cells
Monocyte derived dendritic cells Combined immunodeficiency
Combined immunodeficiency Virus infection
Virus infection Enoxapan
Enoxapan Ptt levels
Ptt levels Contractor acquired property
Contractor acquired property Florida cession date
Florida cession date Plants are sessile
Plants are sessile Dog inherited traits examples
Dog inherited traits examples Acquired traits in animals
Acquired traits in animals What are 5 acquired traits
What are 5 acquired traits Examples of learned traits
Examples of learned traits Acquired physical traits
Acquired physical traits Acquired traits in animals
Acquired traits in animals Inherited and acquired traits
Inherited and acquired traits Systemic acquired resistance in plants
Systemic acquired resistance in plants Causes of hemolysis
Causes of hemolysis Hemolysis symptoms
Hemolysis symptoms Acquired physical traits
Acquired physical traits Biotic examples
Biotic examples Acquired needs theory
Acquired needs theory Acquired hemolytic anemia
Acquired hemolytic anemia Infer how the pigs acquired another case of whiskey.
Infer how the pigs acquired another case of whiskey. Hitchikers thumb
Hitchikers thumb