How to Negotiate with the Sponsor and Keep





















- Slides: 30

How to Negotiate with the Sponsor and Keep Your Budget in the Black Petros G. Okubagzi, MD Washington DC

Petros G. Okubagzi, MD I/we have no real or apparent conflicts of interest to report.

Overview-Good News • Clinical trial spending in 2010~ $25 billion and is expected to reach $28. 5 billion by 2014. • In 2010, the number of clinical trials in the U. S. was 25, 992. Expected to reach 32, 318 in 2014 • In 2009, 34. 8% of sales by medical compaines was spent in domestic R&D. In 2014, projected to reach 48. 8 Marketresearch. com

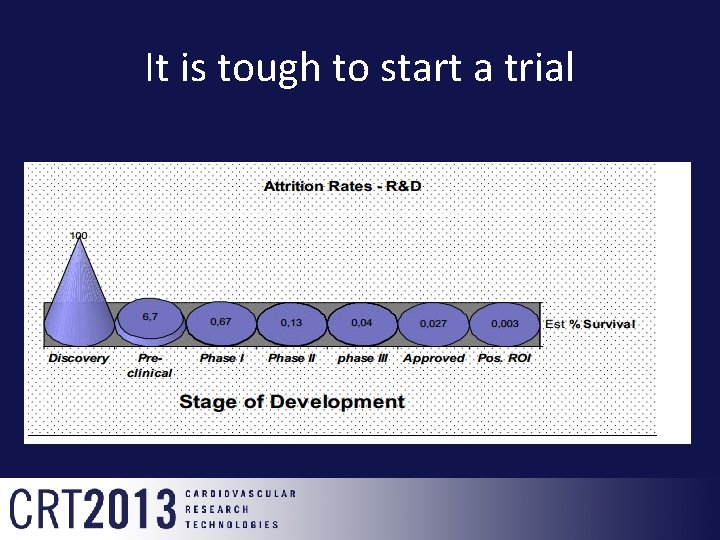
It is tough to start a trial

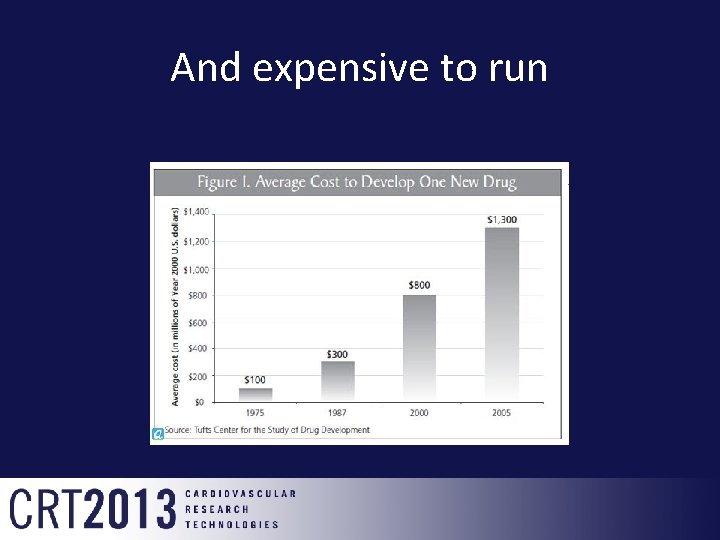
And expensive to run

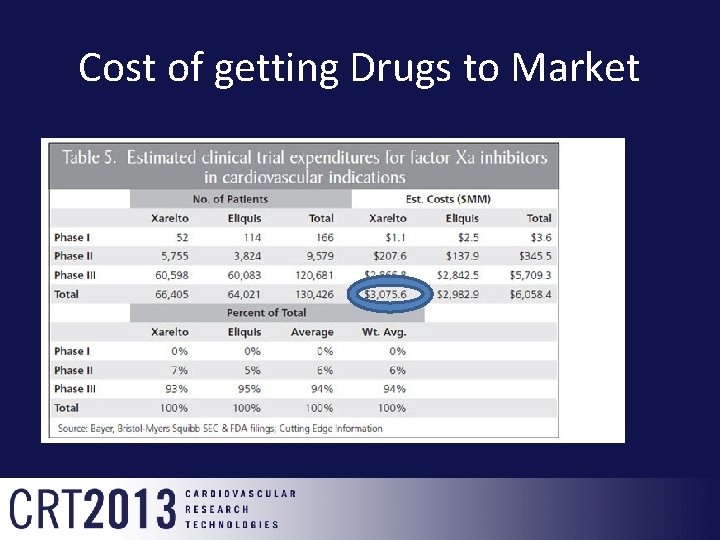
Cost of getting Drugs to Market

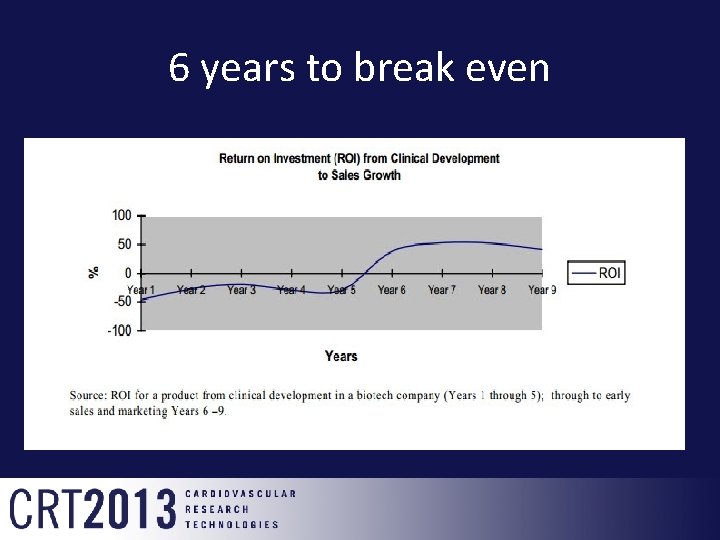
6 years to break even

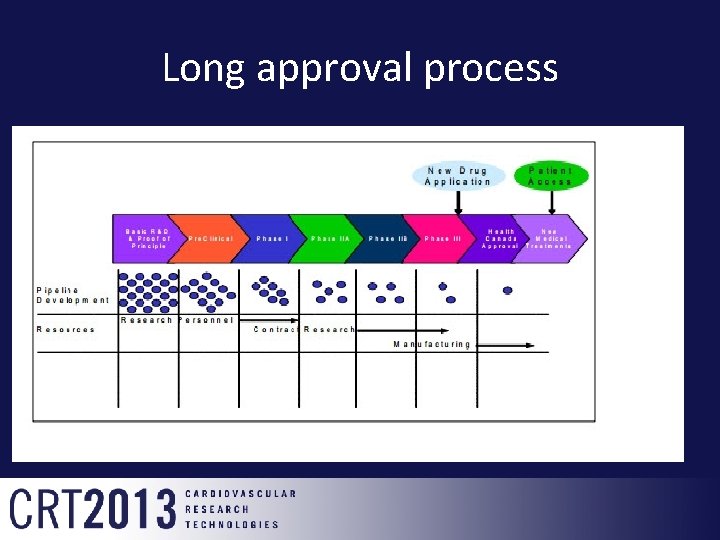
Long approval process

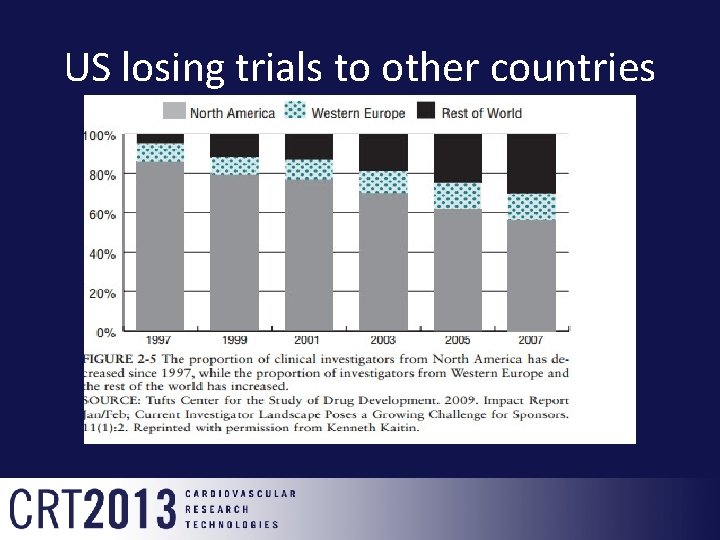
US losing trials to other countries

What happens when Study starts? 1. 2. 3. 4. 5. 6. CDA( Confidentiality Signed) Feasibility Questionnaire/Pre site visit complete Budget template/Contract provided by Sponsor Institutional review( Study team/Hospital) Counter offer provided by Study site Final Contract signed

Basic rules For Survival 1. Continuity 2. Flexibility 3. Information 4. Understanding finances 5. Diversification 6. Planning( Forecasting)

Continuity and Flexibility Have a Stable and experienced team

Information • Keep abreast with FDA rules/new context: Less in person visits? Remote monitoring? “more work for the coordinators” • Medicare changes: what is considered standard of care or Customary “ having staffing with PHD in coding and deciphering essential”

Understand Finances • Negotiate with an Eye on the bigger picture 1. 2. 3. 4. 5. Lots of activities required but not covered Understand the Sponsors expectation Have room for overhead Negotiate for Screen failure patients Provide feedback to sponsor when streched

Keep Relationships

Learn from your mistakes

Diversification • Keep a healthy mix of studies and staff • There is no study “beneath” you. . there is a study you can not and should not do • Listen to the portfolio manager. . Diversify

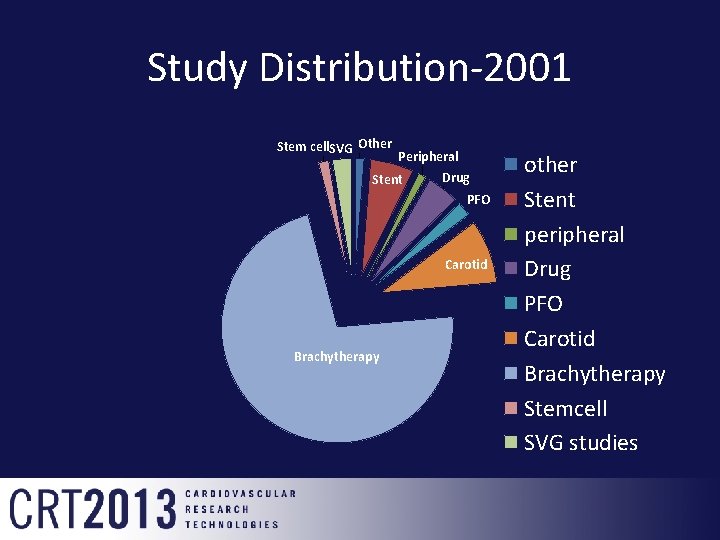
Study Distribution-2001 Stem cell. SVG Other Peripheral Drug Stent PFO Carotid Brachytherapy other Stent peripheral Drug PFO Carotid Brachytherapy Stemcell SVG studies

Study Distribution-2011 Ischemia sensor Drug Valve DAPT Imaging Valve Imaging DAPT Ischemia sensor drug

In Summary • • Have a stable Working group/backbone Keep institutional support Negotiate well Diversify…Diversify. . Keep informed Remember you are a business. . But not a business for profit….

If things are really going bad

Have someone to back you

Avoid Being a One Trick Pony

Planning/Forecasting • Be able to plan a year ahead at least with a sense of the next three-five years • Be sure to take advantage of the sunny days. Do not rest on your laurels • Cross train ( coordinators unfortunately tend to occasionally break, want to go to the beach and have babies) • Watch for the unexpected…

Collaboration Contract Negotiation Medicare Approval Budget Negotiation Enrollment IRB Approval Initiation and start

Getting the Jigsaw together Pathoo gy Resear ch office Clinical Resear ch Coordi nator OHRP Spons or PI FDA IRB JRSC Hospit al Clinical Trials Office Univer sity

Strategy to Maximize Success • Organization • Regular research meetings ¡Daily research staff ¡Discussion of ongoing trials, new trials, etc. • Regular meetings with Investigators ¡Study status, outcomes, violations, amendments ¡Team review of protocols prior to initiation • Study Tracking • Finance Tracking Same thing Giora does

Overview-Challenges • Investigative sites—research centers that conduct clinical research—have continued to shrink and have become more unstable over the past five years. • The most active and experienced investigative sites, who comprise the backbone of the clinical research industry in the U. S. , have seen the sharpest decline.

Overview-Challenges • The proportion of most active FDA-regulated principal investigators (PIs)—those who conduct at least two clinical studies each year —has declined from 29% to less than 23% during the past five years, while the average number of new studies begun by the 60 highest volume sites in the U. S. and Canada fell by 85%. • Centerwatch

Overview-Challenges • Experienced investigative sites, with substantial clinical research infrastructure and high fixed costs, are finding it difficult to stay in business. • Complex protocols, flat study budgets, an onerous regulatory compliance burden and more intense competition for studies are considered to be main difficulties
 Williams and fudge payment
Williams and fudge payment Roosevelt helped negotiate peace between russia and .
Roosevelt helped negotiate peace between russia and . Keep satisfied manage closely monitor keep informed
Keep satisfied manage closely monitor keep informed Threat matrix example
Threat matrix example Keep it secret keep it safe
Keep it secret keep it safe Media have commercial implications
Media have commercial implications Negotiating with sap
Negotiating with sap Job how negotiate salary
Job how negotiate salary Media literacy definition
Media literacy definition And now a word from our sponsor
And now a word from our sponsor Sponsor roadmap
Sponsor roadmap Project sponsor example
Project sponsor example Npqonline
Npqonline Mentor versus sponsor
Mentor versus sponsor Contratto per sponsor pubblicitario
Contratto per sponsor pubblicitario The southside baptist church sponsor a festival
The southside baptist church sponsor a festival Sponsor
Sponsor Bonus sponsor tiens
Bonus sponsor tiens Prosci methodology
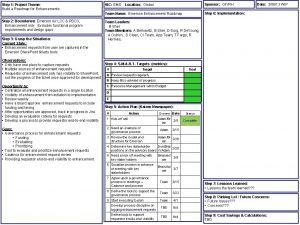
Prosci methodology Npqh task 2 action plan
Npqh task 2 action plan Bonus sponsor
Bonus sponsor Megan jacques
Megan jacques Sponsor letter for visa uk
Sponsor letter for visa uk Henry hudson sponsor country
Henry hudson sponsor country Adult sponsor meaning
Adult sponsor meaning What was de aviles goal
What was de aviles goal Prosci pct assessment
Prosci pct assessment Sponsor
Sponsor Pedro menendez de aviles sponsor
Pedro menendez de aviles sponsor 21 cfr part 312
21 cfr part 312 Sponsor by
Sponsor by