HHV 5014 NUTRACEUTICAL FORMULATION TECHNOLOGY UNIT 7 LIQUID









- Slides: 16

HHV 5014 NUTRACEUTICAL FORMULATION TECHNOLOGY UNIT 7: LIQUID DOSAGE FORM SUB UNIT 7. 5: DISPERSED SYSTEM (SUSPENSION) DAUS FIZA SOFIA IJA

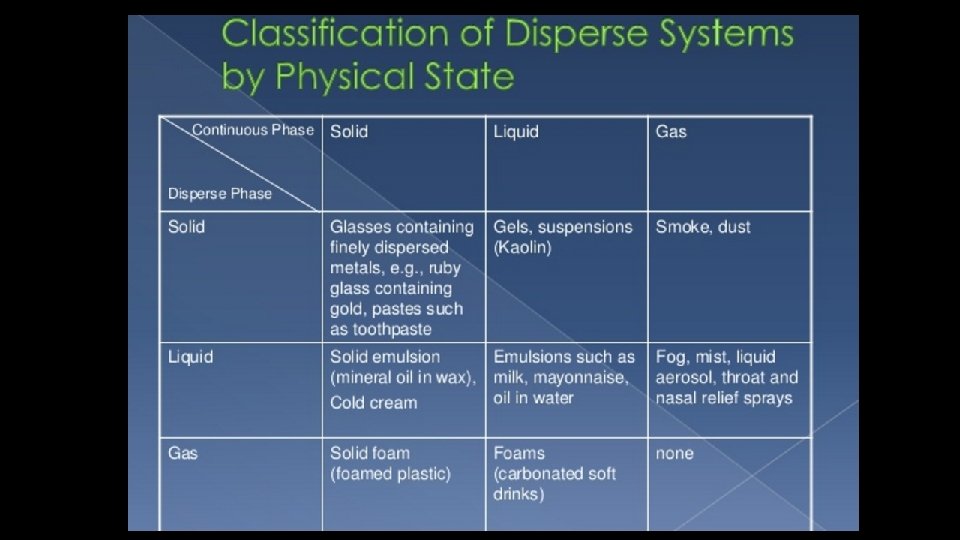
INTRODUCTION • Dispersed system is defined as a heterogeneous two phase system in which internal (dispersed, discontinuous) phase is distributed or dispersed within the continuous (external) phase or vehicle. • The internal and external phase may be solids, liquids and also gases. • Eg: suspensions, emulsions, aerosol.


WHY NEED DISPERSION? For improving product stability Ease of administration and flexibility in administration of a range of doses For masking unpleasant taste of product

SUSPENSIONS • Suspensions defined as preparations containing finely divided drug particles distributed somewhat uniformly throughout a vehicle in which the drug exhibits a minimum degree of solubility.

REASONS FOR SUSPENSION • For improving product stability. • Ease of administration and flexibility in administration of a range of doses.

FEATURES DESIRED IN PHARMACEUTICAL SUSPENSION Particles should settle slowly and should be readily re-dispersed upon shaking of the container. The particle size of the suspension should remain fairly constant throughout long periods of undisturbed standing. The suspension should pour readily and evenly from its container.

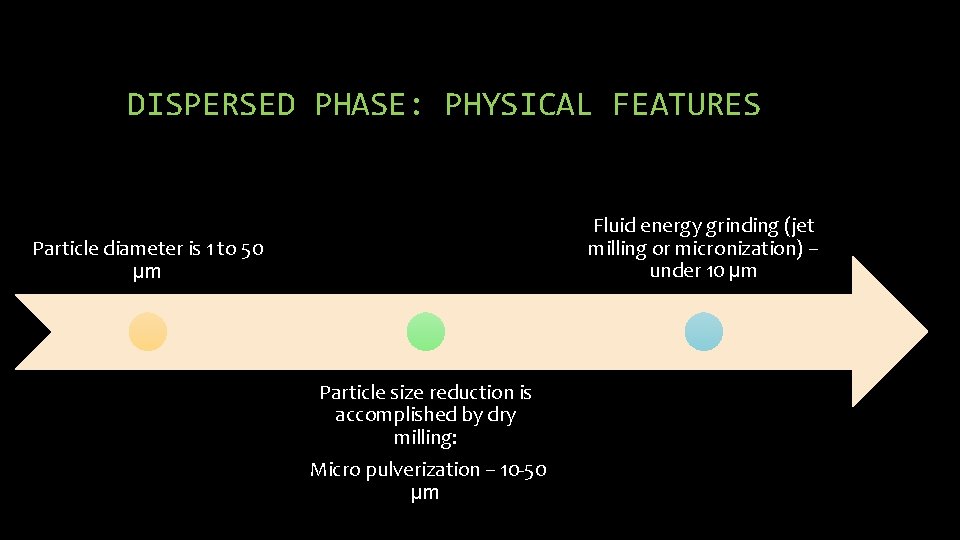
DISPERSED PHASE: PHYSICAL FEATURES Fluid energy grinding (jet milling or micronization) – under 10 µm Particle diameter is 1 to 50 µm Particle size reduction is accomplished by dry milling: Micro pulverization – 10 -50 µm

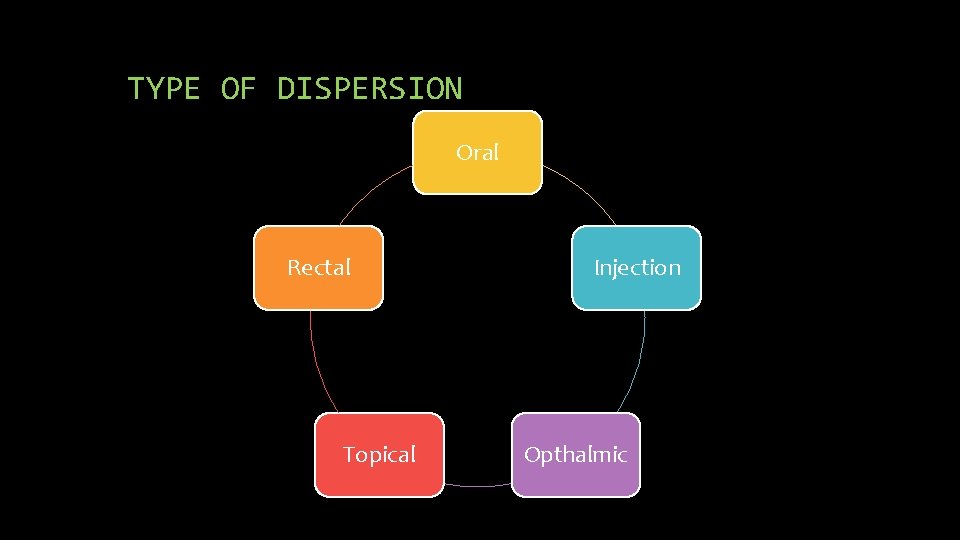
TYPE OF DISPERSION Oral Rectal Topical Injection Opthalmic

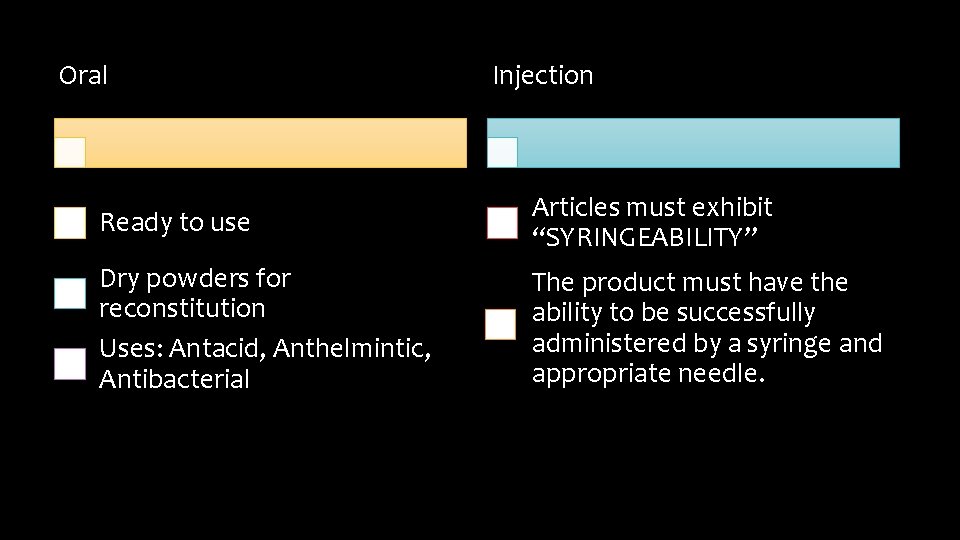
Oral Injection Ready to use Articles must exhibit “SYRINGEABILITY” Dry powders for reconstitution Uses: Antacid, Anthelmintic, Antibacterial The product must have the ability to be successfully administered by a syringe and appropriate needle.

Opthalmic Particle size must not exceed 10 microns Particles must be “impalpable” Topical Fine particles (impalpable) are desired to avoid grittiness when applied to the skin. The smaller the particle size, the greater the covering and protective power of the preparation.

Rectal Barium Sulfate for Suspension May be employed orally or rectally for diagnostic visualization

DISPERSION EQUIPMENT MIXER HIGH SPEED DISPENSER

ROTOR STATOR MIXERS COMBINATION MIXERS

IN LINE MIXER

THANK YOU