NUTRACEUTICAL FORMULATION TECHNOLOGY HHV 5014 UNIT 1 PREFORMULATION
- Slides: 7

NUTRACEUTICAL FORMULATION TECHNOLOGY HHV 5014 UNIT 1: PREFORMULATION & PREPARATION OF RAW MATERIAL 1. 3 TESTING REQUIREMENT FIRDAUS HAFIZAH SOFIA AINIZA

INTRODUCTION • Preformulation testing is the first step in the rational development of dosage forms. • It can be defined as an investigation of physical and chemical property of a drug substance alone and when combined with excipients. • Physiochemical properties are those that can be determined from in vitro experiments.

OBJECTIVE To generate information useful to the formulation in developing most stable and availability dosage form that mass can be produced.

WHY IS PREFORMULATION IMPORTANT? It describes the process of optimizing the delivery of drug thorough determination of physical, chemical properties of new drug molecule that affect drug performance and development of an efficacious stable and safe dosage form. Preformulation studies on a new drug molecule provide useful information for subsequent formulation of a physicochemically stable and biopharmaceutically suitable dosage form.

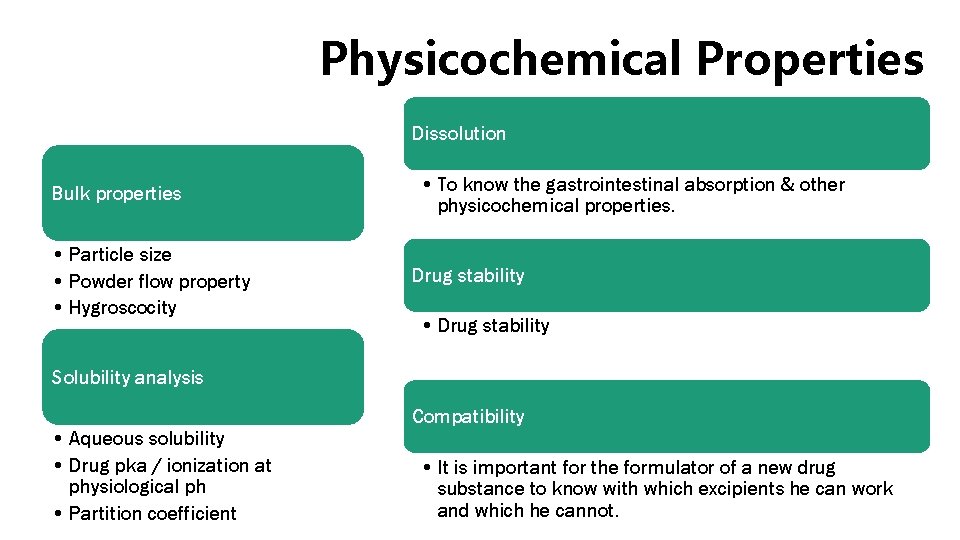
Physicochemical Properties Dissolution Bulk properties • Particle size • Powder flow property • Hygroscocity • To know the gastrointestinal absorption & other physicochemical properties. Drug stability • Drug stability Solubility analysis • Aqueous solubility • Drug pka / ionization at physiological ph • Partition coefficient Compatibility • It is important for the formulator of a new drug substance to know with which excipients he can work and which he cannot.

Analytical Measures Microscopy Spectroscopy Chromatography

REFERENCES 1) Banker GS, Rhodes CT. , Modern pharmaceutics, 4 th ed. New York: Marcel Dekker; 2002. p. 167 -184 2) Alfred Martin. , Physical pharmacy. 4 th ed. New York: Lippincott Williams & Wilkins; 2001. p. 77 -100 3) Remington, The Science and Practice of Pharmacy, 20 th Edition, B. I Publication Pvt Ltd. p. 940 -42 4) Leon Lachman, Herbert AL, Joseph LK. , The theory and practice of industrial pharmacy, 3 rd ed. Bombay: Varghese publishing house; 1990. p. 171 - 196