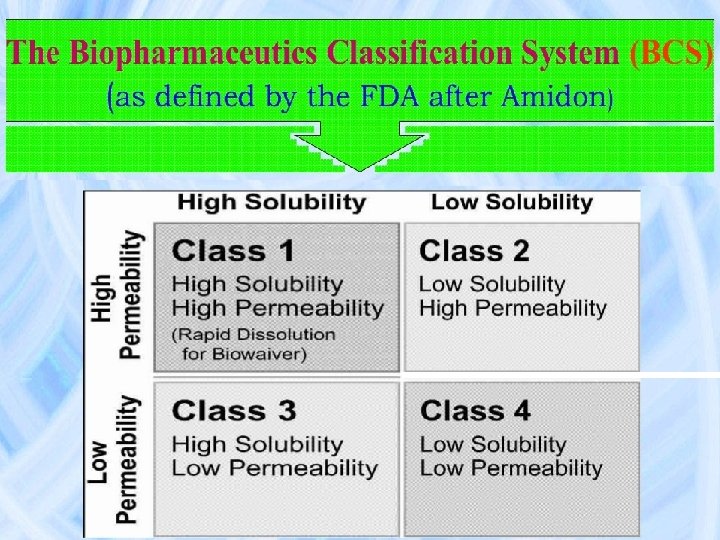
PREFORMULATION STUDIES Bioequivalence Invitro Invivo correlation BCS implication

PREFORMULATION STUDIES

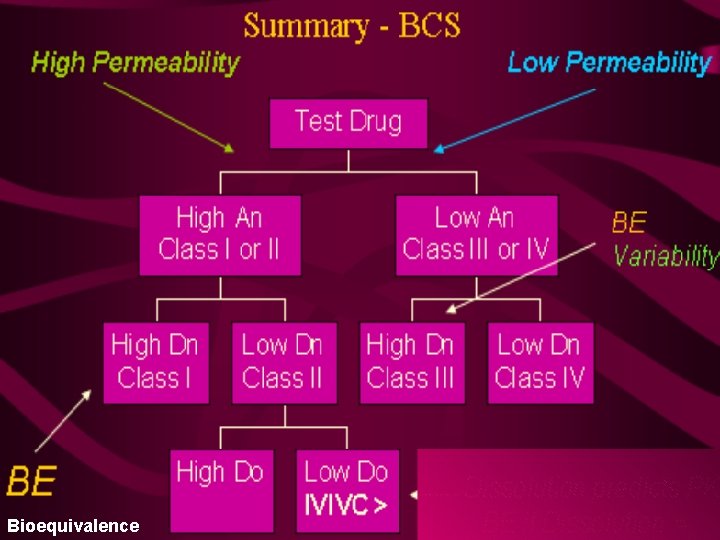
Bioequivalence
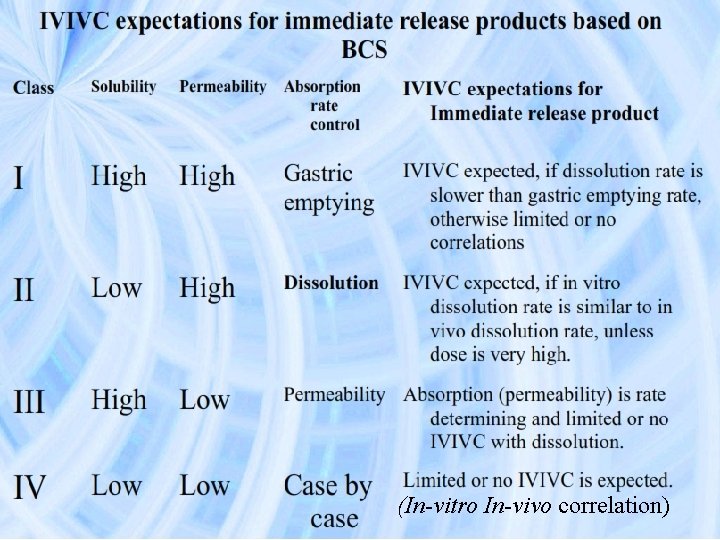
(In-vitro In-vivo correlation)
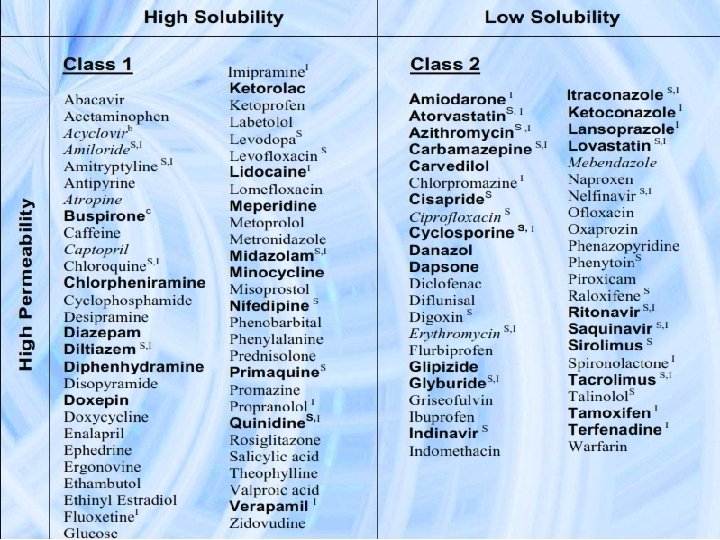
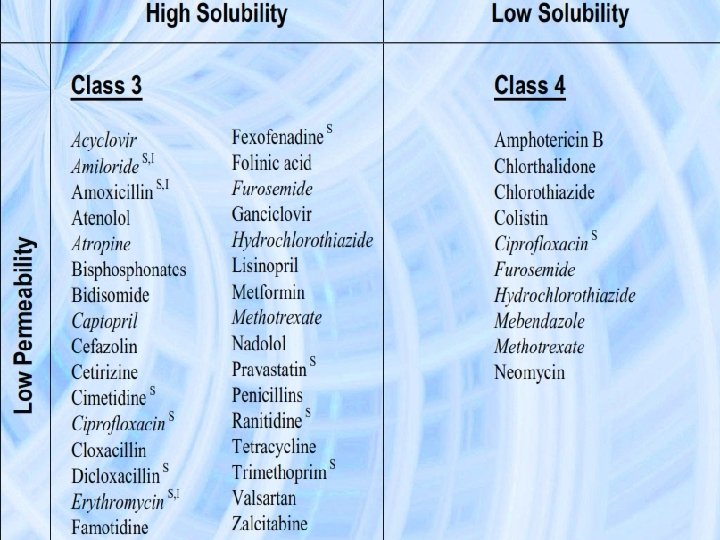

BCS implication in drug Development

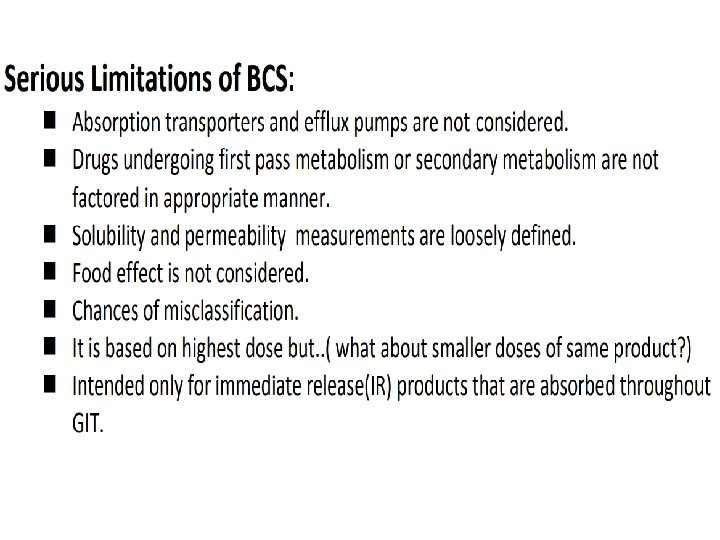

Applications of pre-formulation considerations in development of various dosage forms Following qualities of features are required in drug and dosage form design: 1. Drug would produce specifically desired (therapeutic) effect. 2. Be administered by most desired route at minimal dose and dosing frequency. 3. Drugs have short onset and optimum duration of activity, without any side effect. 4. Would be completely eliminated from body efficiently without any residual effect. 5. Pharmaceutically dosage form should be a. Elegant b. Physically and Chemically stable at various conditions of usage and storage. 1. 2. 3. Biopharmaceutical aspects Therapeutic considerations Drug factors (pre-formulation) aspects to be considered for achieving the above goal
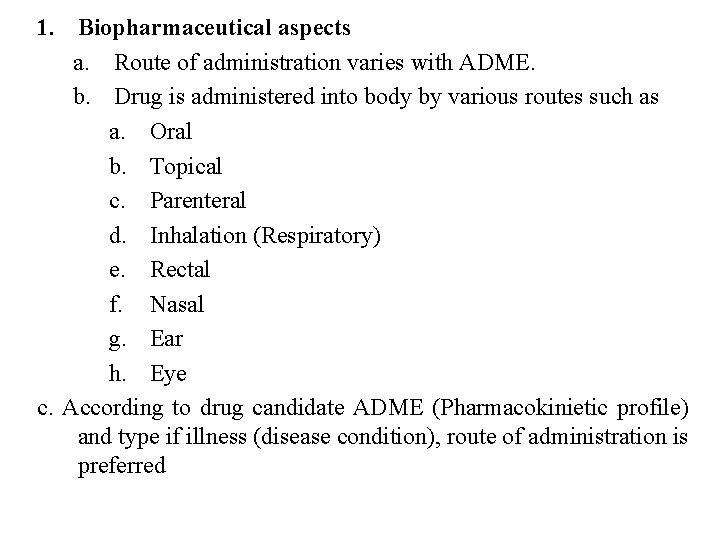
1. Biopharmaceutical aspects a. Route of administration varies with ADME. b. Drug is administered into body by various routes such as a. Oral b. Topical c. Parenteral d. Inhalation (Respiratory) e. Rectal f. Nasal g. Ear h. Eye c. According to drug candidate ADME (Pharmacokinietic profile) and type if illness (disease condition), route of administration is preferred
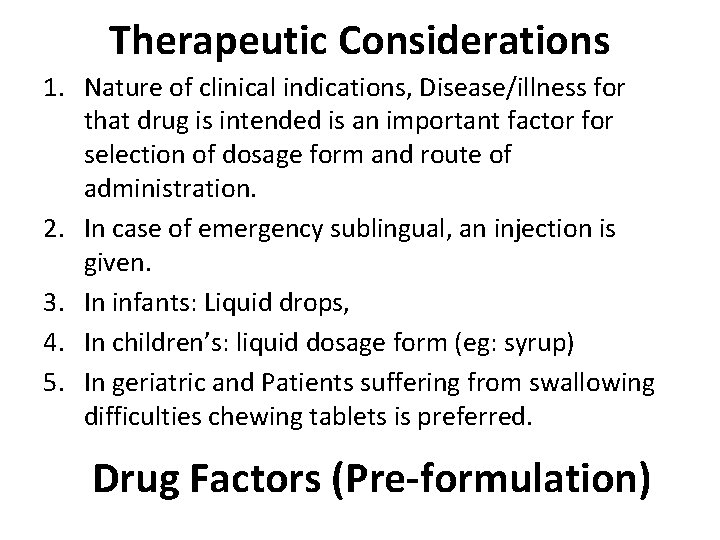
Therapeutic Considerations 1. Nature of clinical indications, Disease/illness for that drug is intended is an important factor for selection of dosage form and route of administration. 2. In case of emergency sublingual, an injection is given. 3. In infants: Liquid drops, 4. In children’s: liquid dosage form (eg: syrup) 5. In geriatric and Patients suffering from swallowing difficulties chewing tablets is preferred. Drug Factors (Pre-formulation)
- Slides: 14