Heat Engine A Real Heat Engine A Perfect








- Slides: 28

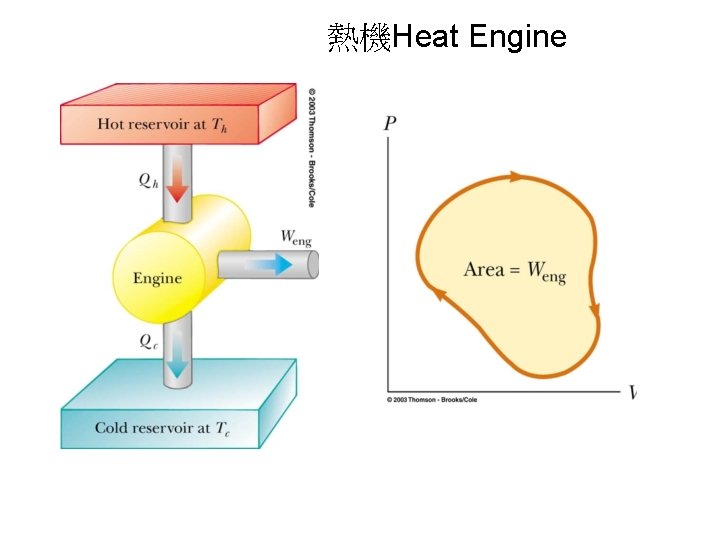
熱機Heat Engine

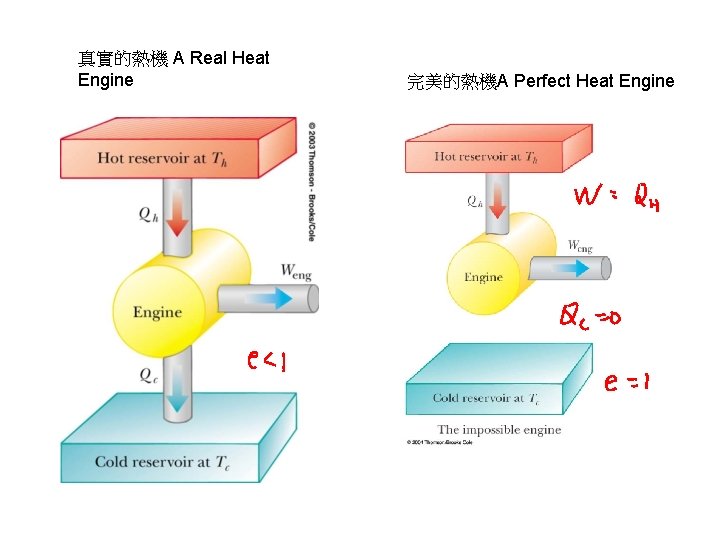
真實的熱機 A Real Heat Engine 完美的熱機A Perfect Heat Engine

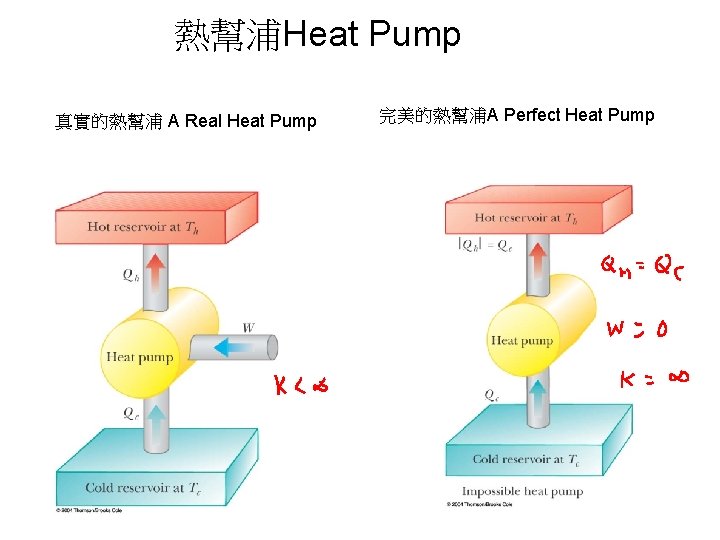
熱幫浦Heat Pump 真實的熱幫浦 A Real Heat Pump 完美的熱幫浦A Perfect Heat Pump

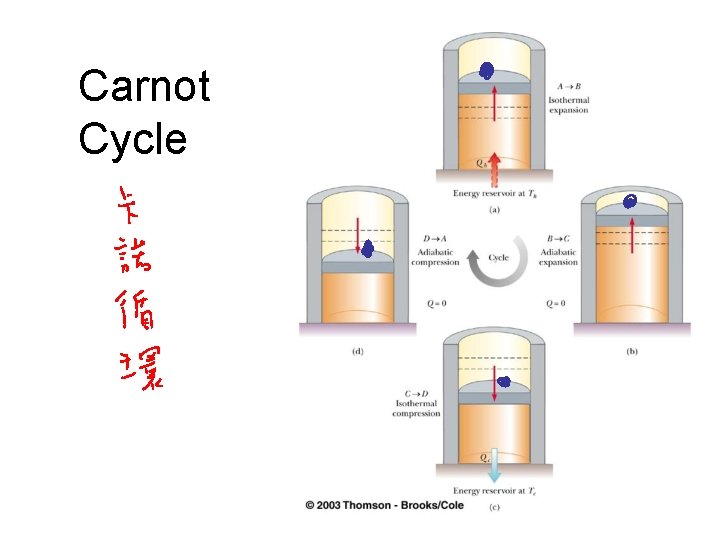
Sadi Carnot • 1796 – 1832 • First to show the quantitative relationship between work and heat • Book reviewed importance of the steam engine

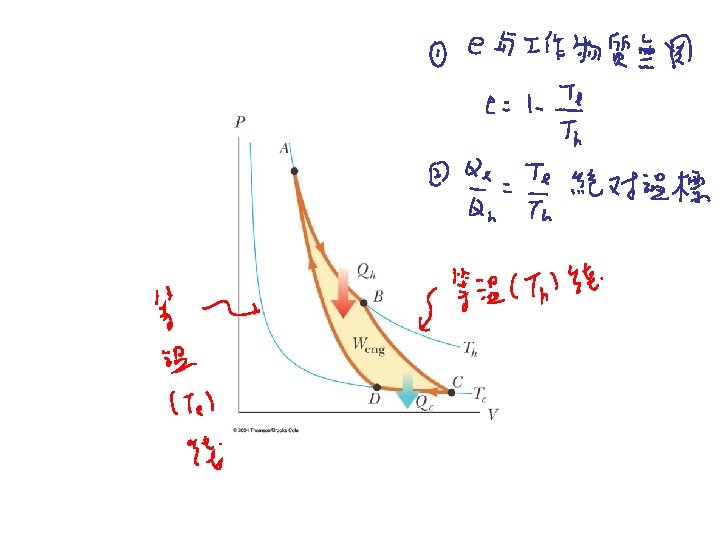
Carnot Cycle


氣體運動論 Kinetic Theory of Gases Ludwid Boltzmann波茲曼 1844 – 1906 貢獻 : 1. 氣體運動論 統計力學 statistical mechanics 2. 電磁學Electromagnetism 3. 熱力學Thermodynamics



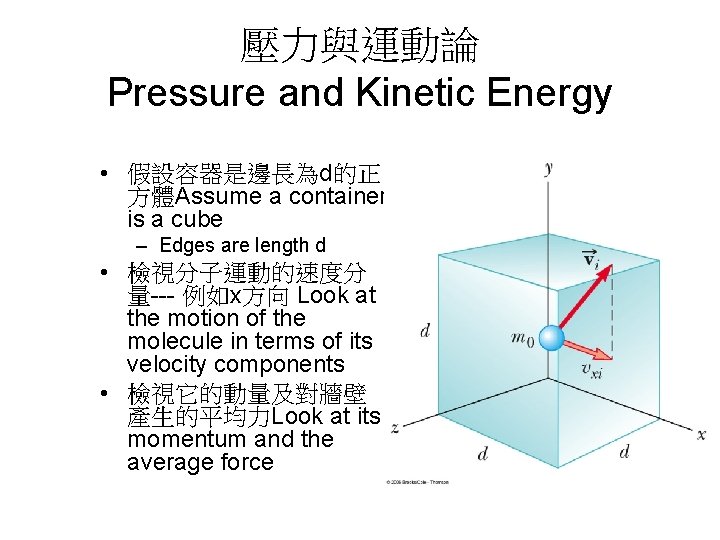
壓力與運動論 Pressure and Kinetic Energy • 假設容器是邊長為d的正 方體Assume a container is a cube – Edges are length d • 檢視分子運動的速度分 量--- 例如x方向 Look at the motion of the molecule in terms of its velocity components • 檢視它的動量及對牆壁 產生的平均力Look at its momentum and the average force

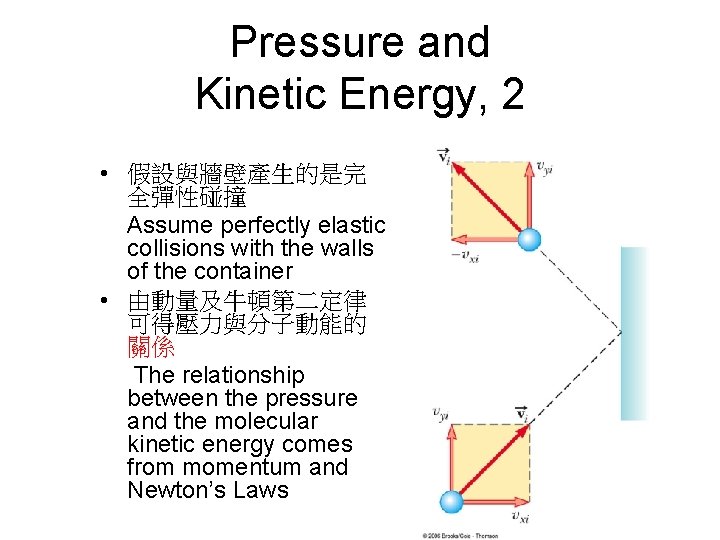
Pressure and Kinetic Energy, 2 • 假設與牆壁產生的是完 全彈性碰撞 Assume perfectly elastic collisions with the walls of the container • 由動量及牛頓第二定律 可得壓力與分子動能的 關係 The relationship between the pressure and the molecular kinetic energy comes from momentum and Newton’s Laws

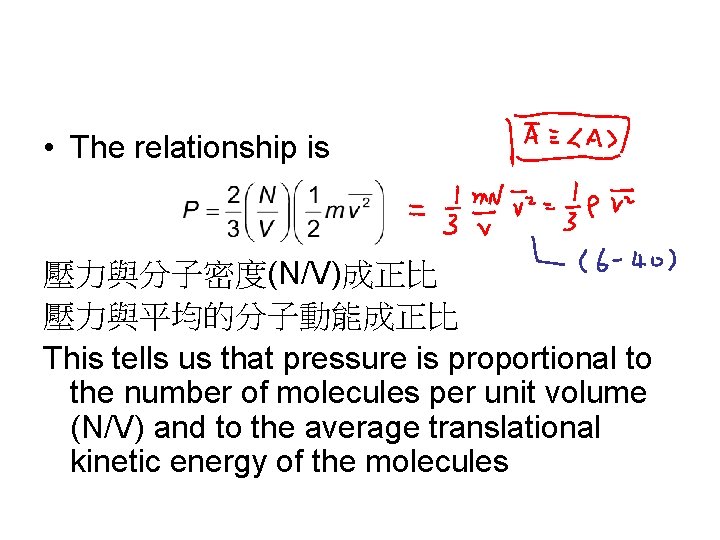
• The relationship is 壓力與分子密度(N/V)成正比 壓力與平均的分子動能成正比 This tells us that pressure is proportional to the number of molecules per unit volume (N/V) and to the average translational kinetic energy of the molecules

如何增加壓力? • 增加N/V One way to increase the pressure is to increase the number of molecules per unit volume • 增加速率(動能) The pressure can also be increased by increasing the speed (kinetic energy) of the molecules

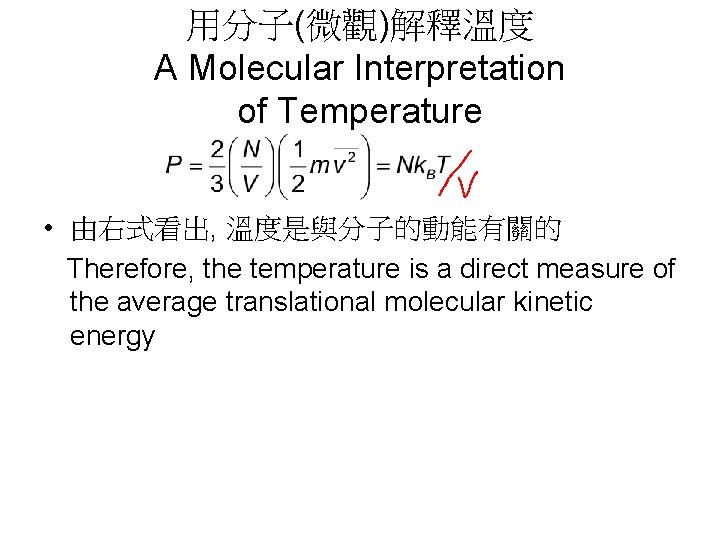
用分子(微觀)解釋溫度 A Molecular Interpretation of Temperature • 由右式看出, 溫度是與分子的動能有關的 Therefore, the temperature is a direct measure of the average translational molecular kinetic energy

• 整理得到 Simplifying the equation relating temperature and kinetic energy gives • 把動能分成三個方向x, y及z, 因此x方向有 This can be applied to each direction, with similar expressions for vy and vz

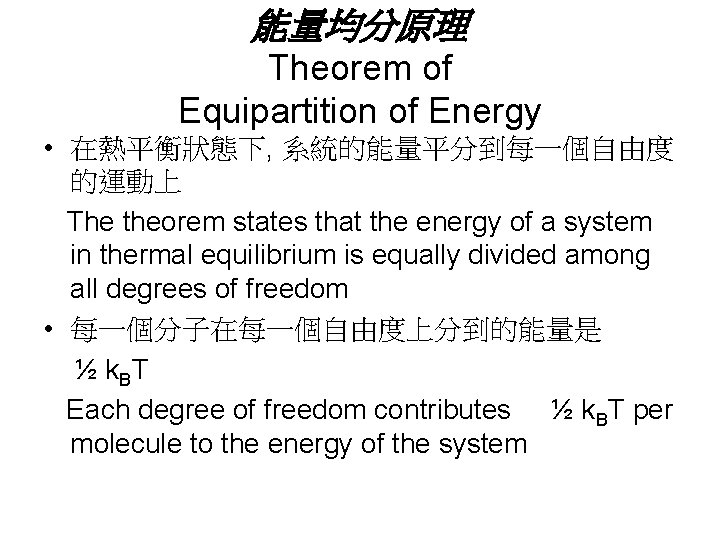
結論 • 分子在每一個自由度方向的運動, 其貢獻的 能量是一樣的 Each translational degree of freedom contributes an equal amount to the energy of the gas – In general, a degree of freedom refers to an independent means by which a molecule can possess energy • 推廣而言, 此即為能量均分原理 A generalization of this result is called theorem of equipartition of energy

能量均分原理 Theorem of Equipartition of Energy • 在熱平衡狀態下, 系統的能量平分到每一個自由度 的運動上 The theorem states that the energy of a system in thermal equilibrium is equally divided among all degrees of freedom • 每一個分子在每一個自由度上分到的能量是 ½ k. BT Each degree of freedom contributes ½ k. BT per molecule to the energy of the system

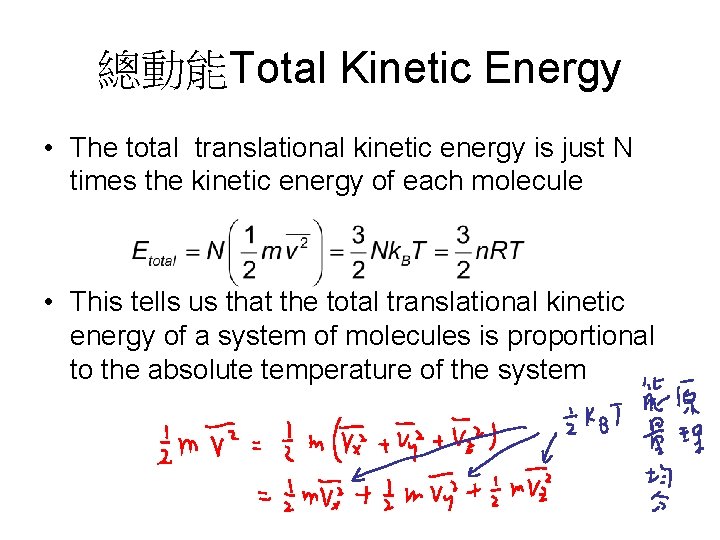
總動能Total Kinetic Energy • The total translational kinetic energy is just N times the kinetic energy of each molecule • This tells us that the total translational kinetic energy of a system of molecules is proportional to the absolute temperature of the system

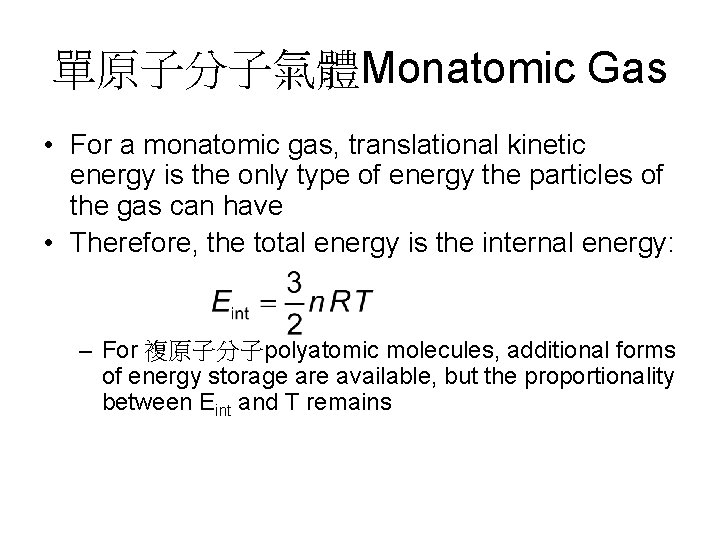
單原子分子氣體Monatomic Gas • For a monatomic gas, translational kinetic energy is the only type of energy the particles of the gas can have • Therefore, the total energy is the internal energy: – For 複原子分子polyatomic molecules, additional forms of energy storage are available, but the proportionality between Eint and T remains

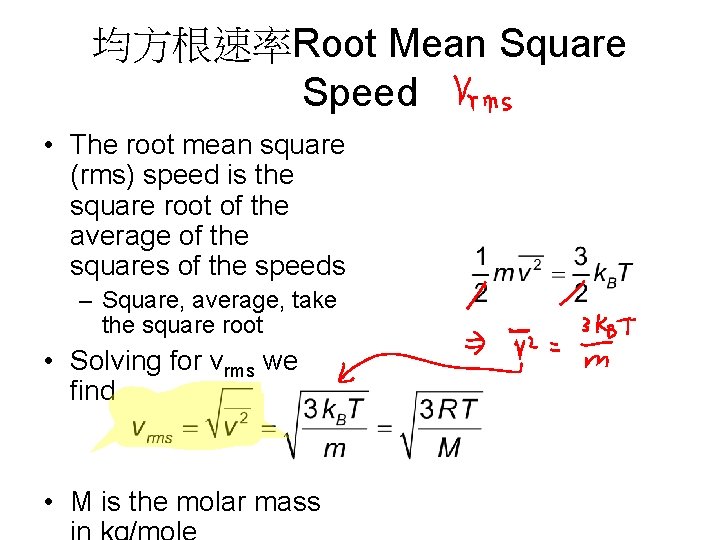
均方根速率Root Mean Square Speed • The root mean square (rms) speed is the square root of the average of the squares of the speeds – Square, average, take the square root • Solving for vrms we find • M is the molar mass

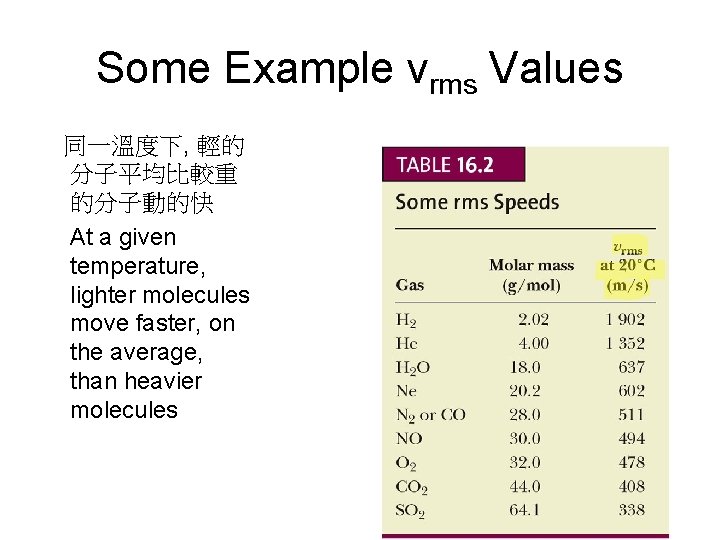
Some Example vrms Values 同一溫度下, 輕的 分子平均比較重 的分子動的快 At a given temperature, lighter molecules move faster, on the average, than heavier molecules

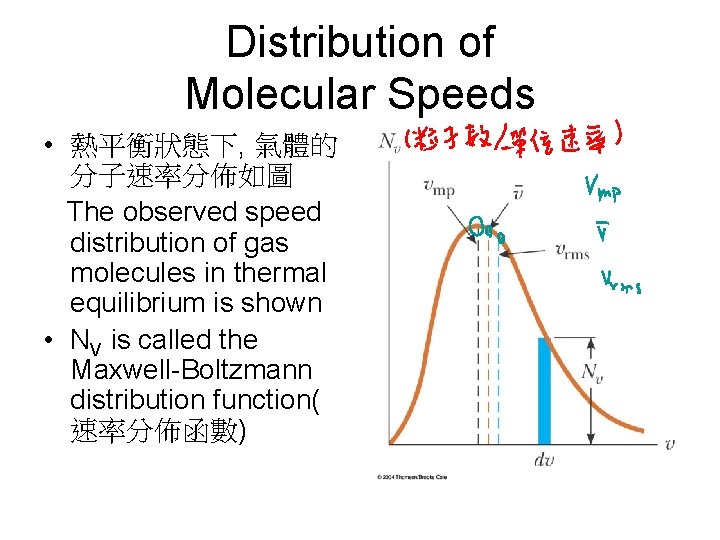
Distribution of Molecular Speeds • 熱平衡狀態下, 氣體的 分子速率分佈如圖 The observed speed distribution of gas molecules in thermal equilibrium is shown • NV is called the Maxwell-Boltzmann distribution function( 速率分佈函數)

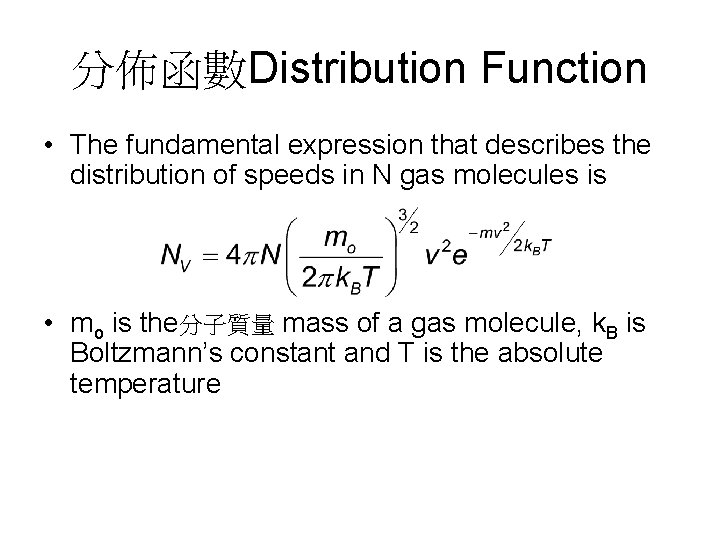
分佈函數Distribution Function • The fundamental expression that describes the distribution of speeds in N gas molecules is • mo is the分子質量 mass of a gas molecule, k. B is Boltzmann’s constant and T is the absolute temperature

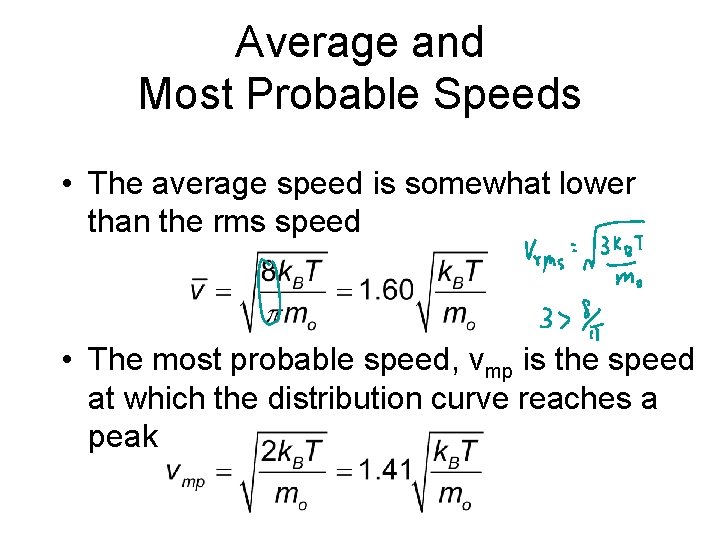
Average and Most Probable Speeds • The average speed is somewhat lower than the rms speed • The most probable speed, vmp is the speed at which the distribution curve reaches a peak

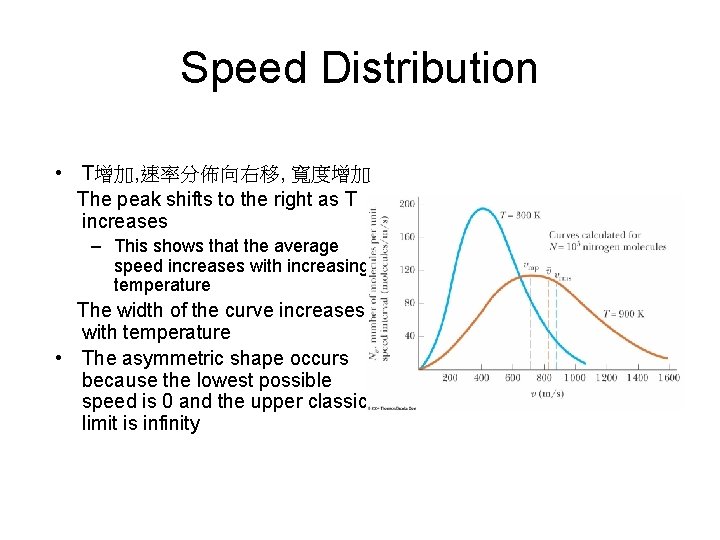
Speed Distribution • T增加, 速率分佈向右移, 寬度增加 The peak shifts to the right as T increases – This shows that the average speed increases with increasing temperature The width of the curve increases with temperature • The asymmetric shape occurs because the lowest possible speed is 0 and the upper classical limit is infinity

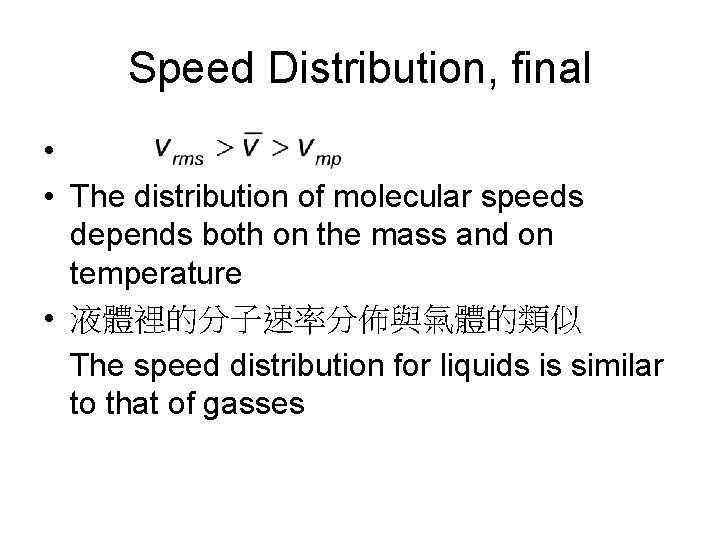
Speed Distribution, final • • The distribution of molecular speeds depends both on the mass and on temperature • 液體裡的分子速率分佈與氣體的類似 The speed distribution for liquids is similar to that of gasses

液體的氣化Evaporation • Some molecules in the liquid are more energetic than others • Some of the faster moving molecules penetrate the surface and leave the liquid – This occurs even before the boiling point is reached • The molecules that escape are those that have enough energy to overcome the attractive forces of the molecules in the liquid phase較大動能的分子能脫離液體的吸引力 • The molecules left behind have lower kinetic energies留下較 少動能的分子, 液體分子的平均速率因而減小, 對應較低之溫度 • 因此Therefore, 氣化evaporation is a 冷卻過程cooling process

大氣Atmosphere • For such a huge volume of gas as the atmosphere, the assumption of a uniform temperature throughout the gas is not valid • There are variations in temperature – Over the surface of the Earth – At different heights in the atmosphere