Half Life Calculations Aim Nuke 5 e How




- Slides: 10

Half Life Calculations Aim Nuke 5 e – How long does radioactivity last?

Half Life The time it takes for a naturally occurring radioisotope to decay half of its amount This is more of a statistical value – the probability of a radioisotope breaking down in a given half life period is 50% See the PHET Simulation at right for more Half life is constant - cannot be altered by: Temperature Pressure Chemical changes The amount See Reference Table N for selected half lives and decay modes

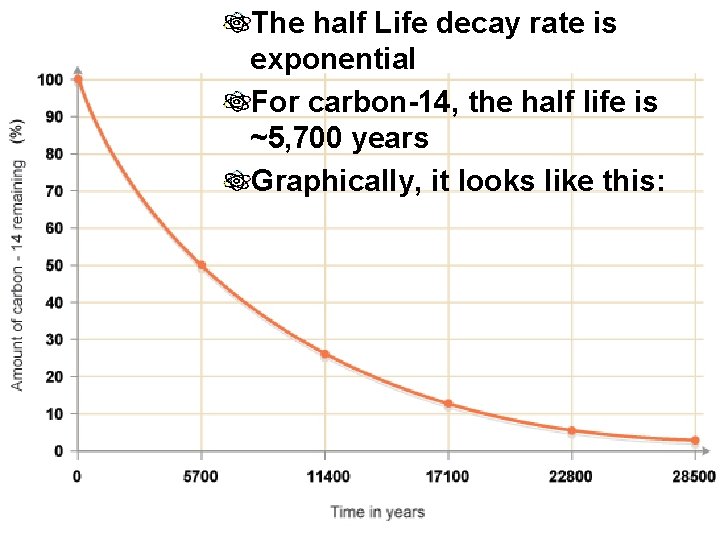
The half Life decay rate is exponential For carbon-14, the half life is ~5, 700 years Graphically, it looks like this:

Half Life In half life calculations, two values often need to be determined The number of half lives that passed (t/T) And the fraction remaining = ( ½ ) t/T Where t = total time and T = half-life period There are four types of half life reactions you will need to calculate: 1. Going into the future – where there is less radioisotope 2. Going into the past – where you started with more than you have now 3. Determine the half life period of an unknown radioisotope 4. Radiometric dating – how old is the sample or object under investigation

Half Life Problem 1 The half-life of Rn-222 (a carcinogenic house pollutant) is 3. 8 days. If today your basement contains 20. 0 grams of Rn-222, how much will remain after 19 days assuming no more leaks in? 1. First calculate the number of half lives that pass in 19 days • 19 days / 3. 8 days per half life • = 5 half lives passed 2. What fraction would remain after 5 half lives? • ( ½ )5 = ½ x ½ x ½ = 1/32 remaining • 20. 0 grams x 1/32 = 0. 625 grams remain

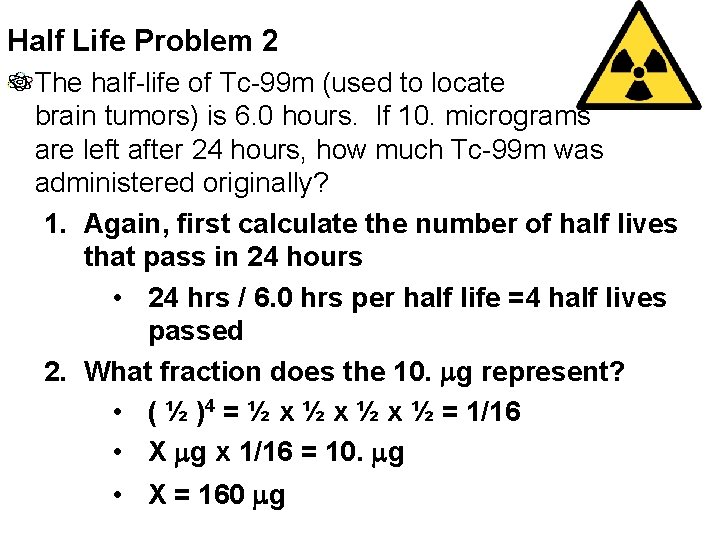
Half Life Problem 2 The half-life of Tc-99 m (used to locate brain tumors) is 6. 0 hours. If 10. micrograms are left after 24 hours, how much Tc-99 m was administered originally? 1. Again, first calculate the number of half lives that pass in 24 hours • 24 hrs / 6. 0 hrs per half life =4 half lives passed 2. What fraction does the 10. mg represent? • ( ½ )4 = ½ x ½ x ½ = 1/16 • X mg x 1/16 = 10. mg • X = 160 mg

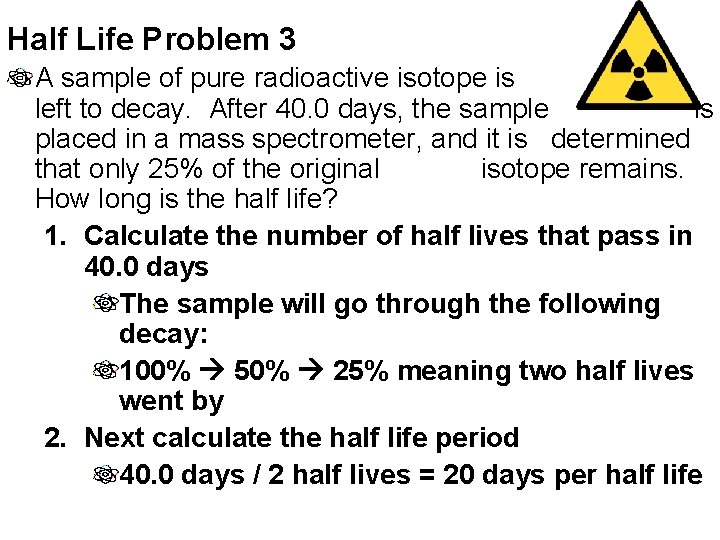
Half Life Problem 3 A sample of pure radioactive isotope is left to decay. After 40. 0 days, the sample is placed in a mass spectrometer, and it is determined that only 25% of the original isotope remains. How long is the half life? 1. Calculate the number of half lives that pass in 40. 0 days The sample will go through the following decay: 100% 50% 25% meaning two half lives went by 2. Next calculate the half life period 40. 0 days / 2 half lives = 20 days per half life

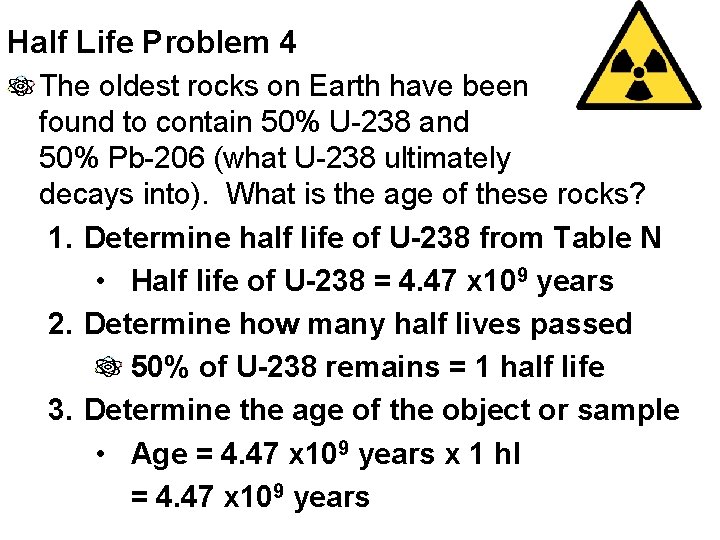
Half Life Problem 4 The oldest rocks on Earth have been found to contain 50% U-238 and 50% Pb-206 (what U-238 ultimately decays into). What is the age of these rocks? 1. Determine half life of U-238 from Table N • Half life of U-238 = 4. 47 x 109 years 2. Determine how many half lives passed 50% of U-238 remains = 1 half life 3. Determine the age of the object or sample • Age = 4. 47 x 109 years x 1 hl = 4. 47 x 109 years

Uses of Radioisotopes Radioactive Dating By comparing the ratio of parent radioisotopes to daughter nuclei Age of various materials can be determined Examples • C-14 vs C-12 – archeological evidence • U-238 vs Pb-206 – rock formations – Much longer half - lives

Other Uses of Radioisotopes Biological Tracers Radioisotopes used to study biochemical reactions Most have short half-lives C-14 and O-18 – photosynthesis and cellular respiration reactions in organisms I-131 – medical diagnosis of thyroid disorders Radiation Therapy Iodine-131 – also used to also destroy cancers Technetium-99 - used in bone scans Cobalt-60 – used to destroy cancers