General Chemistry CHEM 101 310 Dr Mohamed ElNewehy




- Slides: 14

General Chemistry CHEM 101 (3+1+0) Dr. Mohamed El-Newehy http: //fac. ksu. edu. sa/melnewehy

Chapter 4 Reactions in Aqueous Solutions

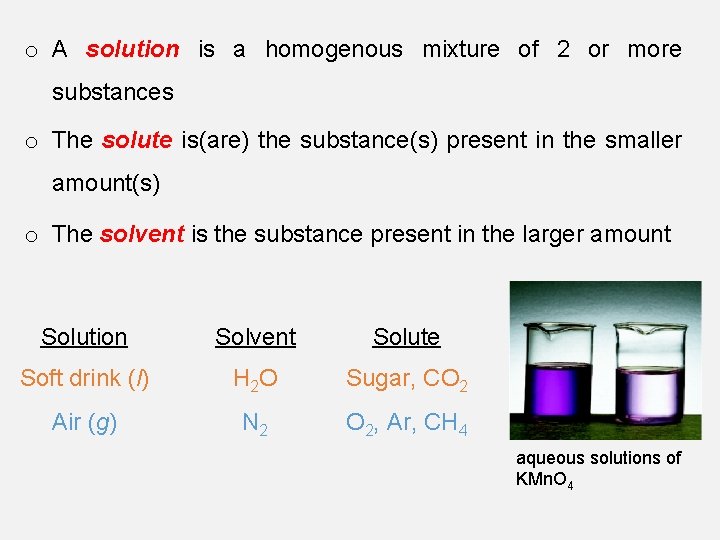
o A solution is a homogenous mixture of 2 or more substances o The solute is(are) the substance(s) present in the smaller amount(s) o The solvent is the substance present in the larger amount Solution Solvent Solute Soft drink (l) H 2 O Sugar, CO 2 Air (g) N 2 O 2, Ar, CH 4 aqueous solutions of KMn. O 4

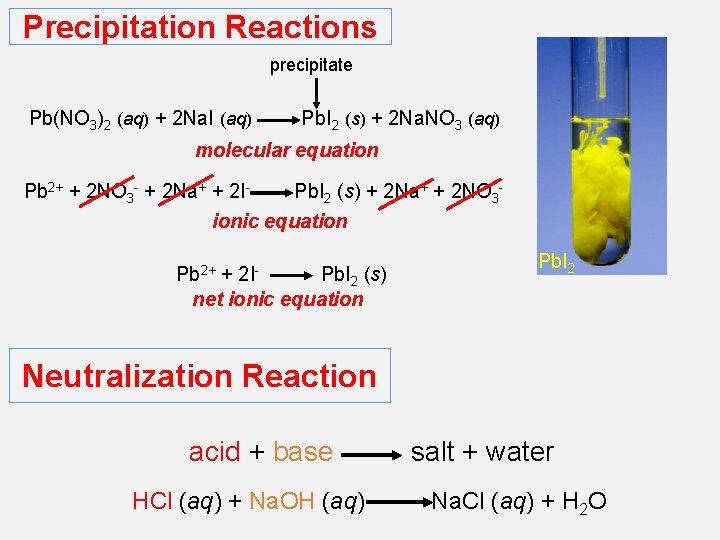
Precipitation Reactions precipitate Pb(NO 3)2 (aq) + 2 Na. I (aq) Pb. I 2 (s) + 2 Na. NO 3 (aq) molecular equation Pb 2+ + 2 NO 3 - + 2 Na+ + 2 I- Pb. I 2 (s) + 2 Na+ + 2 NO 3 - ionic equation Pb 2+ 2 I- + Pb. I 2 (s) net ionic equation Pb. I 2 Neutralization Reaction acid + base HCl (aq) + Na. OH (aq) salt + water Na. Cl (aq) + H 2 O

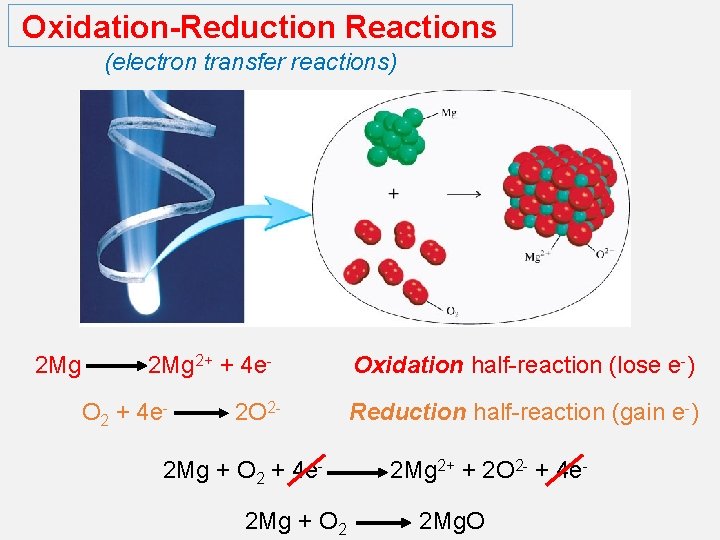
Oxidation-Reduction Reactions (electron transfer reactions) 2 Mg 2+ + 4 e. O 2 + 4 e- 2 O 2 - 2 Mg + O 2 + 4 e 2 Mg + O 2 Oxidation half-reaction (lose e-) Reduction half-reaction (gain e-) 2 Mg 2+ + 2 O 2 - + 4 e 2 Mg. O

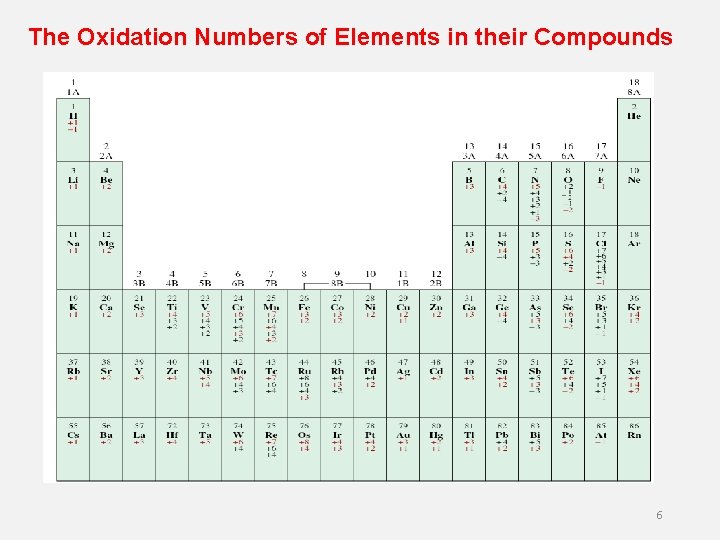
The Oxidation Numbers of Elements in their Compounds 6

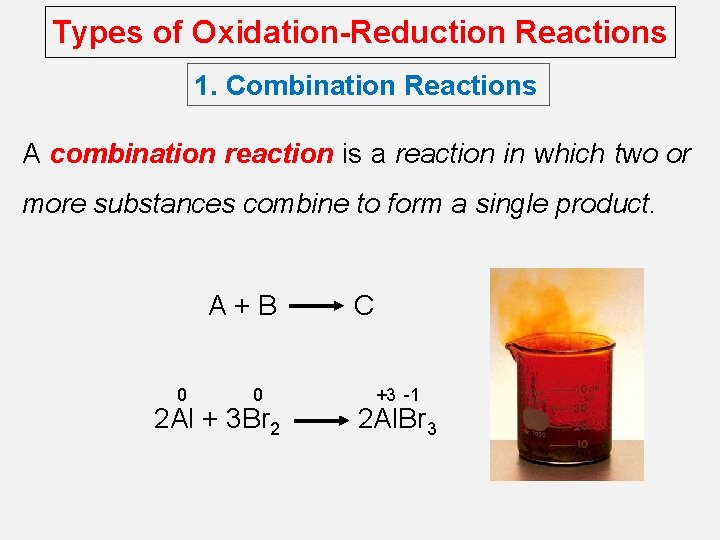
Types of Oxidation-Reduction Reactions 1. Combination Reactions A combination reaction is a reaction in which two or more substances combine to form a single product. A+B 0 0 2 Al + 3 Br 2 C +3 -1 2 Al. Br 3

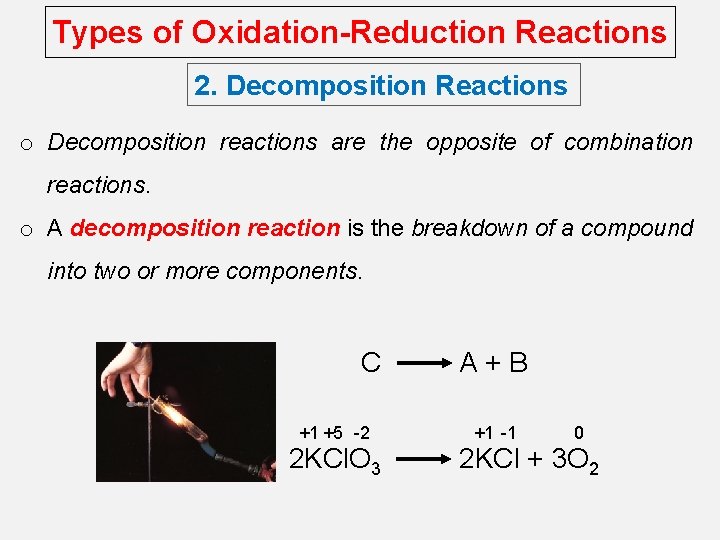
Types of Oxidation-Reduction Reactions 2. Decomposition Reactions o Decomposition reactions are the opposite of combination reactions. o A decomposition reaction is the breakdown of a compound into two or more components. C +1 +5 -2 2 KCl. O 3 A+B +1 -1 0 2 KCl + 3 O 2

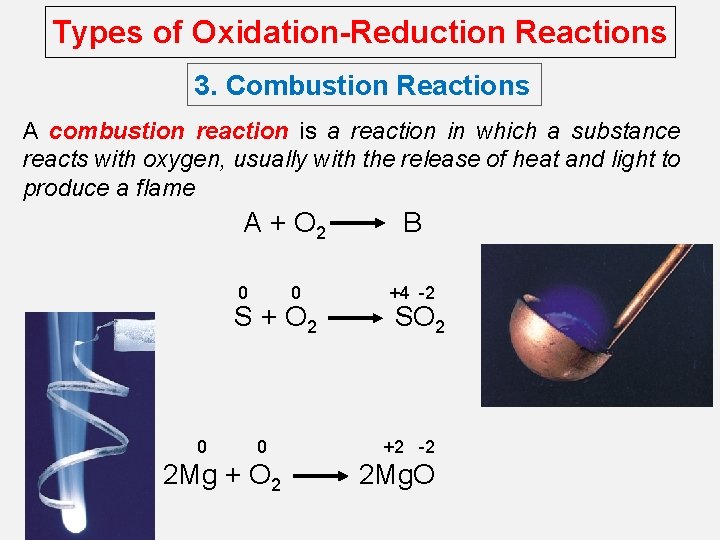
Types of Oxidation-Reduction Reactions 3. Combustion Reactions A combustion reaction is a reaction in which a substance reacts with oxygen, usually with the release of heat and light to produce a flame A + O 2 0 0 S + O 2 0 0 2 Mg + O 2 B +4 -2 SO 2 +2 -2 2 Mg. O

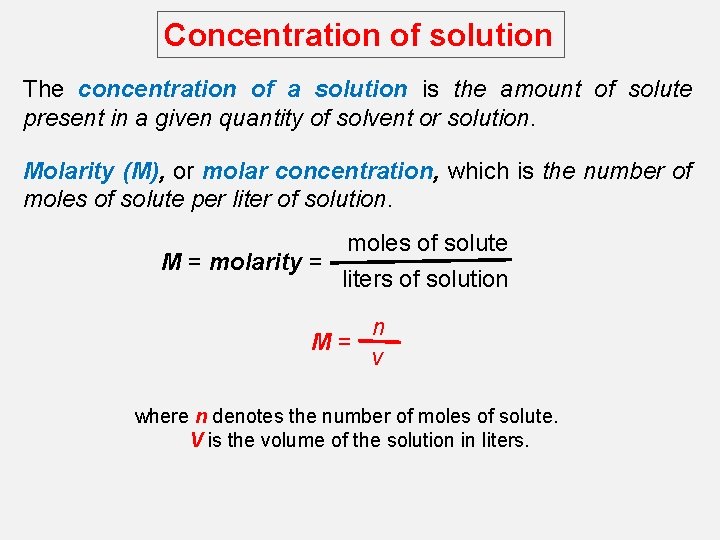
Concentration of solution The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. Molarity (M), or molar concentration, which is the number of moles of solute per liter of solution. M = molarity = moles of solute liters of solution n M= v where n denotes the number of moles of solute. V is the volume of the solution in liters.

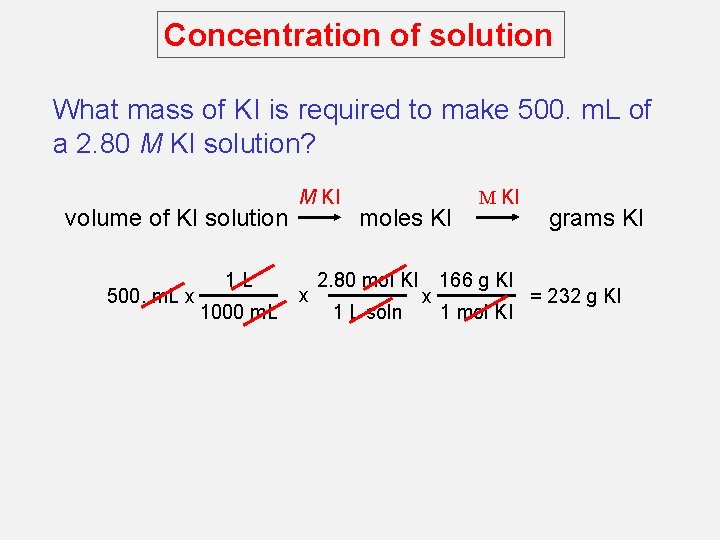
Concentration of solution What mass of KI is required to make 500. m. L of a 2. 80 M KI solution? volume of KI solution 500. m. L x 1 L 1000 m. L M KI x moles KI 2. 80 mol KI 1 L soln x M KI 166 g KI 1 mol KI grams KI = 232 g KI

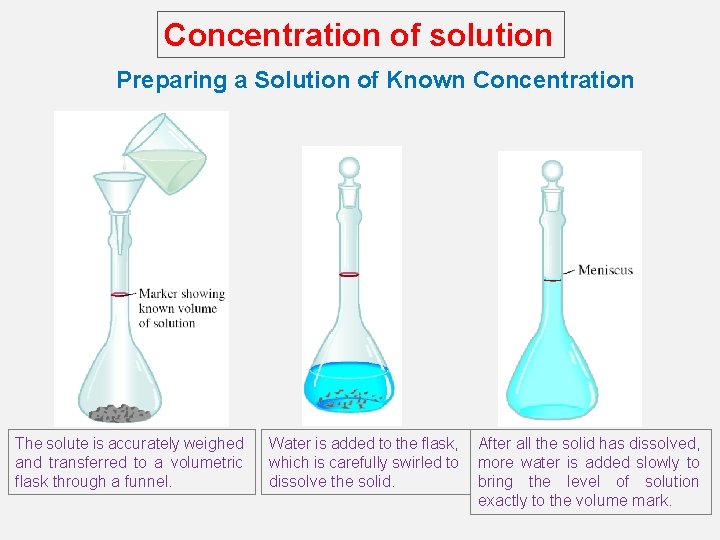
Concentration of solution Preparing a Solution of Known Concentration The solute is accurately weighed and transferred to a volumetric flask through a funnel. Water is added to the flask, which is carefully swirled to dissolve the solid. After all the solid has dissolved, more water is added slowly to bring the level of solution exactly to the volume mark.

Concentration of solution

Concentration of solution