Freeze Drying or Lyophilization Certain food stuff which









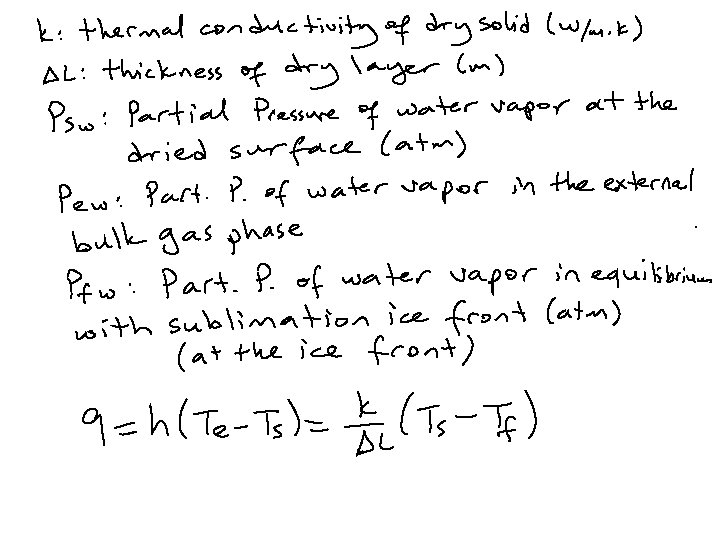

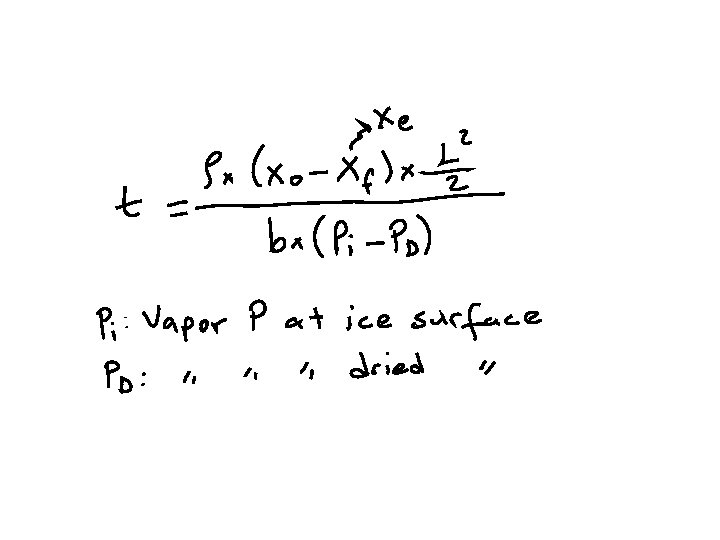

- Slides: 24

Freeze Drying or Lyophilization Certain food stuff which can not be heated even to moderate temperatures in ordinary drying, may be freeze dried. The substance to be dried is usually frozen by very cold air. In freeze drying the water is as a vapor by sublimation from the frozen material in a vacuum chamber. After the moisture sublimes to a vapor, it is removed by mechanical vacuum pumps or steam jet ejector.

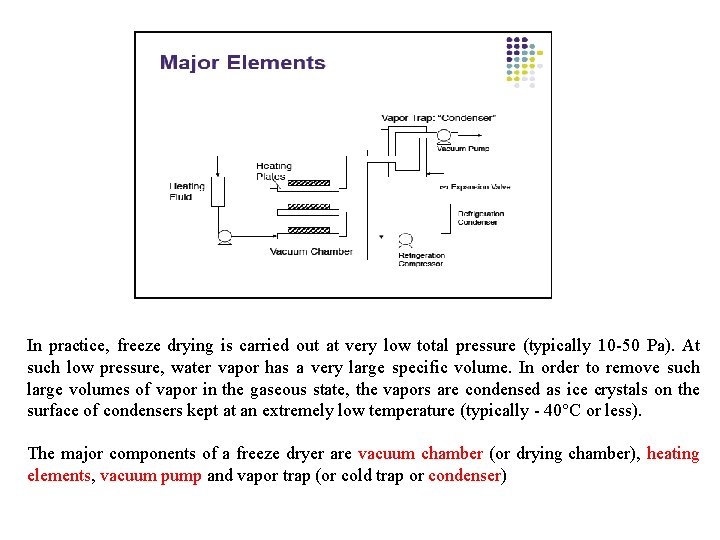
In practice, freeze drying is carried out at very low total pressure (typically 10 -50 Pa). At such low pressure, water vapor has a very large specific volume. In order to remove such large volumes of vapor in the gaseous state, the vapors are condensed as ice crystals on the surface of condensers kept at an extremely low temperature (typically - 40°C or less). The major components of a freeze dryer are vacuum chamber (or drying chamber), heating elements, vacuum pump and vapor trap (or cold trap or condenser)

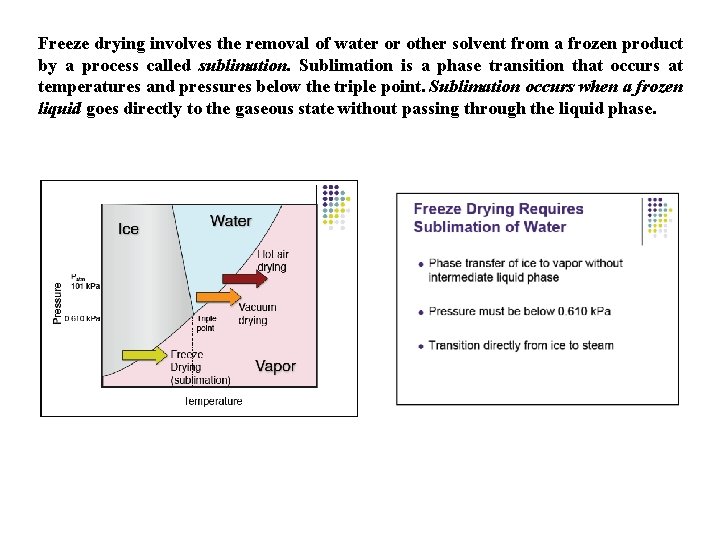
Freeze drying involves the removal of water or other solvent from a frozen product by a process called sublimation. Sublimation is a phase transition that occurs at temperatures and pressures below the triple point. Sublimation occurs when a frozen liquid goes directly to the gaseous state without passing through the liquid phase.

The freeze drying process consists of three stages: 1. Prefreezing, 2. Primary drying, and 3. Secondary drying. 1. Prefreezing: Since freeze drying is a change in state, material to be freeze dried must first be adequately prefrozen. When the aqueous suspension is cooled, changes occur in the solute concentrations of the product matrix. And as cooling proceeds, the water is separated from the solutes as it changes to ice, creating more concentrated areas of solute. These pockets of concentrated materials have a lower freezing temperature than the water. Although a product may appear to be frozen because of all the ice present, in actuality it is not completely frozen until all of the solute in the suspension is frozen. Only when all of the eutectic mixture is frozen is the suspension properly frozen. This is called the eutectic temperature.

It is very important in freeze drying to prefreeze the product to below the eutectic temperature before beginning the freeze drying process. Small pockets of unfrozen material remaining in the product expand compromise the structural stability of the freeze dried product. The second type of frozen product is a suspension that undergoes glass formation during the freezing process. Instead of forming eutectics, the entire suspension becomes increasingly viscous as the temperature is lowered. Finally the product freezes at the glass transition point forming a vitreous solid. This type of product is extremely difficult to freeze dry. 2. Primary drying: After prefreezing the product, conditions must be established in which ice can be removed from the frozen product via sublimation, resulting in a dry, structurally intact product. This requires very careful control of the two parameters, temperature and pressure, involved in the freeze drying system. The rate of sublimation of ice from a frozen product depends upon the difference in vapor pressure of the product compared to the vapor pressure of the ice collector. Molecules migrate from the higher pressure sample to a lower pressure area. Since vapor pressure is related to temperature, it is necessary that the product temperature is warmer than the cold trap (ice collector) temperature.

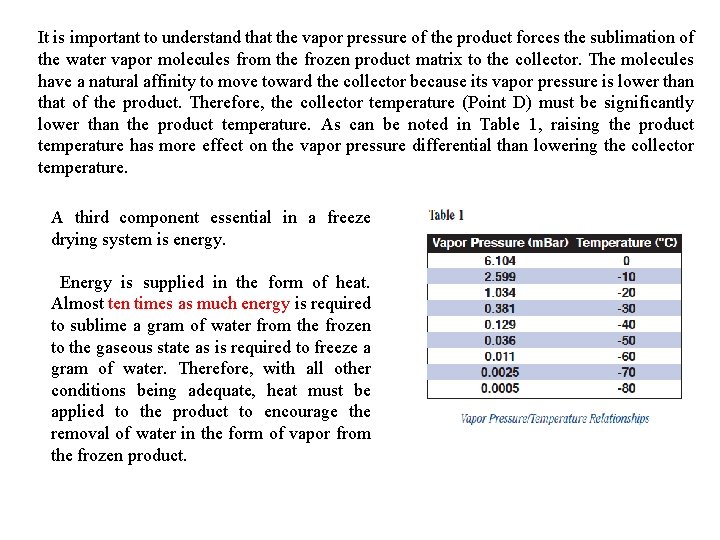
It is important to understand that the vapor pressure of the product forces the sublimation of the water vapor molecules from the frozen product matrix to the collector. The molecules have a natural affinity to move toward the collector because its vapor pressure is lower than that of the product. Therefore, the collector temperature (Point D) must be significantly lower than the product temperature. As can be noted in Table 1, raising the product temperature has more effect on the vapor pressure differential than lowering the collector temperature. A third component essential in a freeze drying system is energy. Energy is supplied in the form of heat. Almost ten times as much energy is required to sublime a gram of water from the frozen to the gaseous state as is required to freeze a gram of water. Therefore, with all other conditions being adequate, heat must be applied to the product to encourage the removal of water in the form of vapor from the frozen product.

Secondary drying: After primary freeze drying is complete, and all ice has sublimed, bound moisture is still present in the product. The product appears dry, but the residual moisture content may be as high as 7 -8%. Continued drying is necessary at the warmer temperature to reduce the residual moisture content to optimum values. This process is called isothermal desorption as the bound water is desorbed from the product. Secondary drying is normally continued at a product temperature higher than ambient but compatible with the sensitivity of the product. All other conditions, such as pressure and collector temperature, remain the same. Because the process is desorptive, the vacuum should be as low as possible (no elevated pressure) and the collector temperature as cold as can be attained. Secondary drying is usually carried out for approximately 1/3 to 1/2 the time required for primary drying. The remaining water after secondary drying is controlled between 2% to 5%. How Freeze Drying Works Refer to the phase diagram, The product is first cooled to below its eutectic temperature (Point A). The collector is cooled to a temperature approximately 20°C cooler than the product temperature, generally around -50 to -105° C. The product should be freeze dried at a temperature slightly lower than its eutectic or collapse temperature (Point B) since the colder the product, the longer the time required to complete primary drying, and the colder the collector temperature required to adequately freeze dry the product.

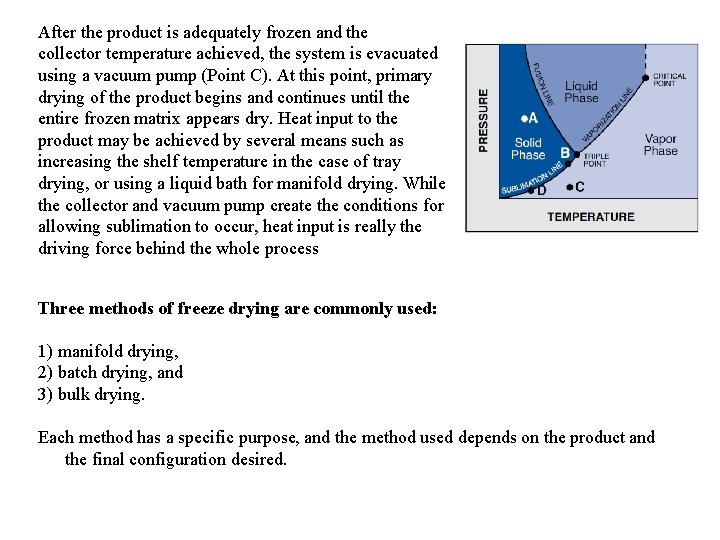
After the product is adequately frozen and the collector temperature achieved, the system is evacuated using a vacuum pump (Point C). At this point, primary drying of the product begins and continues until the entire frozen matrix appears dry. Heat input to the product may be achieved by several means such as increasing the shelf temperature in the case of tray drying, or using a liquid bath for manifold drying. While the collector and vacuum pump create the conditions for allowing sublimation to occur, heat input is really the driving force behind the whole process Three methods of freeze drying are commonly used: 1) manifold drying, 2) batch drying, and 3) bulk drying. Each method has a specific purpose, and the method used depends on the product and the final configuration desired.

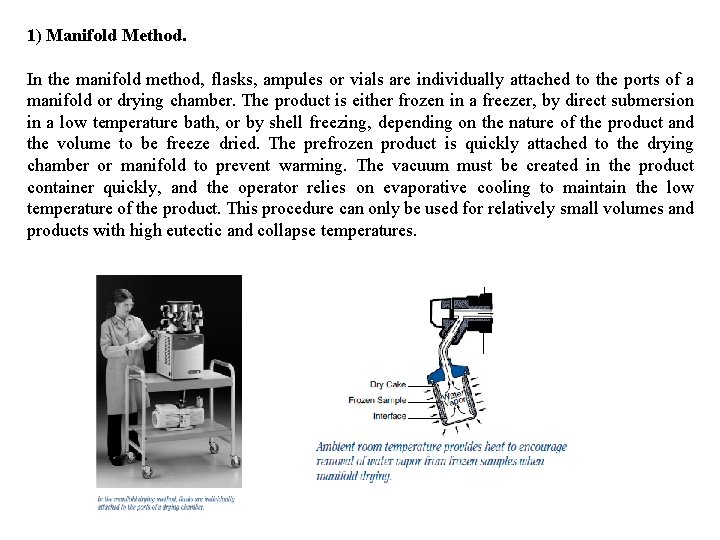
1) Manifold Method. In the manifold method, flasks, ampules or vials are individually attached to the ports of a manifold or drying chamber. The product is either frozen in a freezer, by direct submersion in a low temperature bath, or by shell freezing, depending on the nature of the product and the volume to be freeze dried. The prefrozen product is quickly attached to the drying chamber or manifold to prevent warming. The vacuum must be created in the product container quickly, and the operator relies on evaporative cooling to maintain the low temperature of the product. This procedure can only be used for relatively small volumes and products with high eutectic and collapse temperatures.

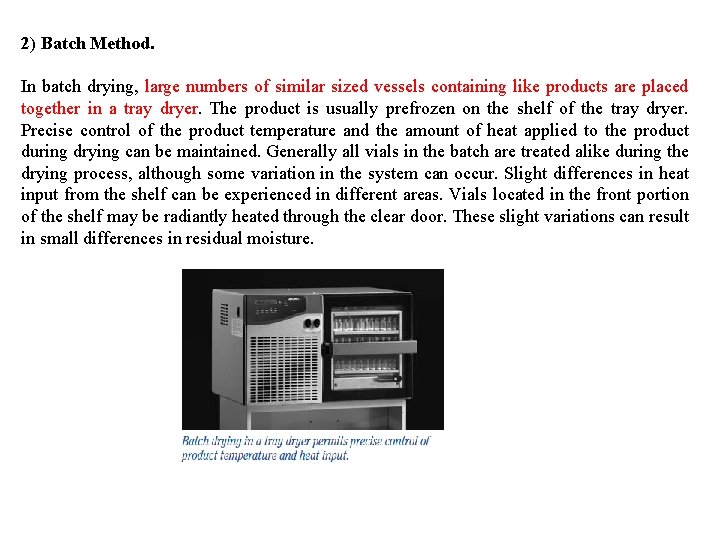
2) Batch Method. In batch drying, large numbers of similar sized vessels containing like products are placed together in a tray dryer. The product is usually prefrozen on the shelf of the tray dryer. Precise control of the product temperature and the amount of heat applied to the product during drying can be maintained. Generally all vials in the batch are treated alike during the drying process, although some variation in the system can occur. Slight differences in heat input from the shelf can be experienced in different areas. Vials located in the front portion of the shelf may be radiantly heated through the clear door. These slight variations can result in small differences in residual moisture.

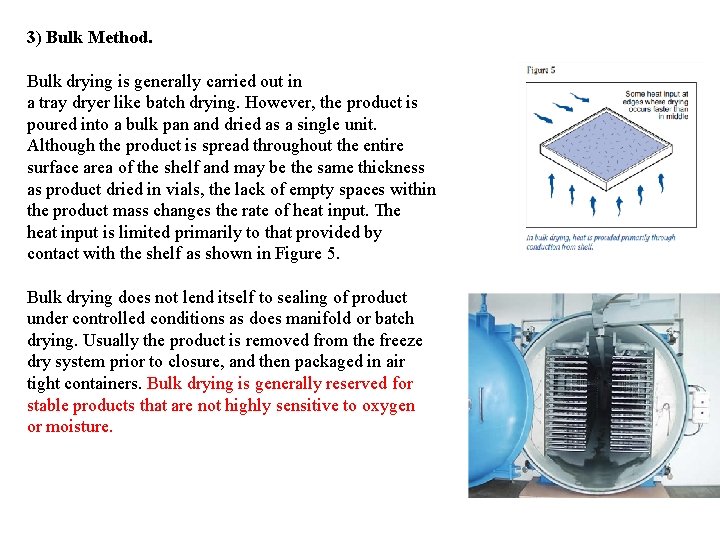
3) Bulk Method. Bulk drying is generally carried out in a tray dryer like batch drying. However, the product is poured into a bulk pan and dried as a single unit. Although the product is spread throughout the entire surface area of the shelf and may be the same thickness as product dried in vials, the lack of empty spaces within the product mass changes the rate of heat input. The heat input is limited primarily to that provided by contact with the shelf as shown in Figure 5. Bulk drying does not lend itself to sealing of product under controlled conditions as does manifold or batch drying. Usually the product is removed from the freeze dry system prior to closure, and then packaged in air tight containers. Bulk drying is generally reserved for stable products that are not highly sensitive to oxygen or moisture.

The advantages of freeze-dried food The process at low temperature and low pressure makes freeze drying an effective way to keep the color, smell, flavor and heat-sensitive nutrients of food and also eliminates the surface hardening of food. Freeze-dried food is porous and easy to be rehydrated and instantly dissolved. It can be consumed directly or after rehydration. Since freeze-dried food contains very low moisture content, it has relatively small density and is easy to be transported. The freeze-dried food can be preserved at room temperature for a long time. The disadvantages of freeze-dried food If exposed directly to air, freeze-dried food will be rehydrated quickly and a series of chemical reactions will happen, resulting in the deterioration of food. The freeze-dried products have to be vacuum-packaging or vacuum-nitrogen charged packaging. The packaging materials should be waterproof. During transportation and sale process, freeze-dried food is easy to be powdered or cracked for its loose porous structure. Freeze-drying is a time-consuming (Lengthy process time) and energy-consuming process, which lead to higher product costs of freeze-dried Freeze drying system includes vacuum and refrigeration equipments. The initial costs are relatively high.

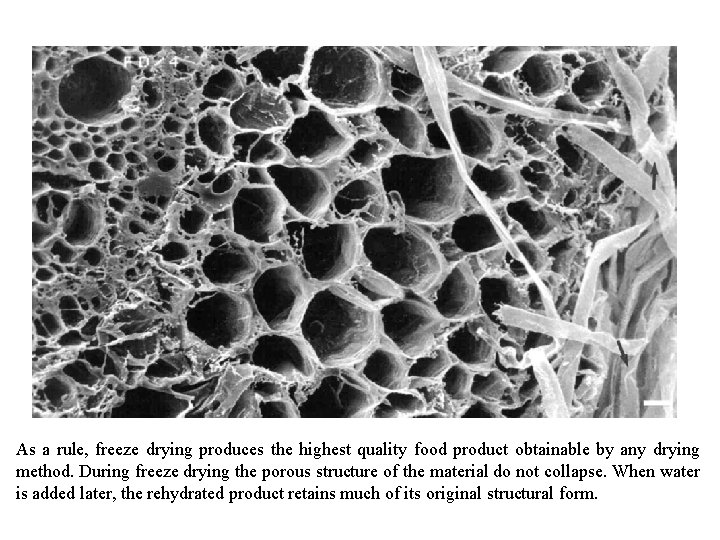
As a rule, freeze drying produces the highest quality food product obtainable by any drying method. During freeze drying the porous structure of the material do not collapse. When water is added later, the rehydrated product retains much of its original structural form.



Techniques for food freeze-drying • • preparation and pretreatment freeze-drying packaging and storage Rehydration Preparation and pretreatment 1. Pretreatment of fruits and vegetables • Pretreatments of fruits and vegetables include sorting the raw materials, cleaning, peeling, trimming, slicing, blanching and so on. Sorting and cleaning are essential processes for all types of fruits and vegetables. The other pretreatment processes are selected depending on the types of raw materials and products. 2. Pretreament of meat products • • The pretreament of meat products includes removing excessive fat, cutting, cooking, or adding the necessary antioxidants. When the freeze drying time is too long and the temperature is too high, the fat in meat products may melt and result in off-odor and coloring. The melted fat will plug the pores in the freeze-dried layer and block the escape of vapor during the drying process. Therefore, the fat should be removed before freeze-drying. Generally, meat without bone is used for freeze drying because the drying of bones need very long time and the dried bones are useless.

• • • Cutting surface should be perpendicular to the direction of the muscle fibers in order to accelerate the sublimation of ice. Cutting can be done when the meat is fresh, and cutting can also be performed when the meat is semi-frozen or totally frozen to obtain good rehydration of the freeze dried meat. In most cases, the freeze-dried meat is consumed directly, so the meat products should be cooked before freeze-driying. In the pretreatment process, anti-oxidants can be added (such as glutathione, ascorbic acid and tocopherol to prevent fat, protein and pigment from oxidation. 3. Pretreatment of liquid foods • Liquid foods can be classified into natural and extract liquid foods. Natural liquid foods include milk, egg yolk, egg white, and so on. Extract liquid foods include fruit juice, vegetable juice, tea, coffee, and so on. • The pretreatment of liquid foods include sterilization, concentration, granulation, adding antioxidant and anti-caking agents. The methods of sterilization are types of foods dependent, for example, milk can be sterilized by pasteurization.

Preventing moisture absorption and oxidation The freeze-dried granular powders will cake and agglomerate when absorbing moisture. It is necessary to prevent freeze-dried food from moisture absorption because freeze-dried food will lose its intrinsic properties, or even deteriorate after absorbing moisture. Packaging materials • Generally, packaging material is required: safe, non-toxic, waterproof, airtight, lightproof, high mechanical strength, suitable for mechanical filling and sealing, easy for transportation. • Brown glass bottles or metal cans should be used if the products are required long-term storage (more than two years). In recent years, the aluminum foil bags partially replaced the metal and glass container. The aluminum foil bags have a variety of laminating methods, such as, aluminum foil + Polyethylene; cellophane + Polyethylene + aluminum foil + polyethylene; paper + Polyethylene + aluminum foil + Paper + polyethylene; cellophane + Polyethylene + aluminum foil + paper + Polyethylene. • No matter which packaging materials are used, the oxygen in the containers must be replaced with nitrogen. The concentration of residual oxygen should be less than 2%. The desiccants inside the package can prevent moisture absorption of the freeze-dried food. The common desiccants are activated carbon, silica gel and so on. • The packaged freeze-dried food should be stored at the possible low temperature environment. If the storage temperature is too high, the shelf life will be shortened due to the caking, discoloring, collapse.

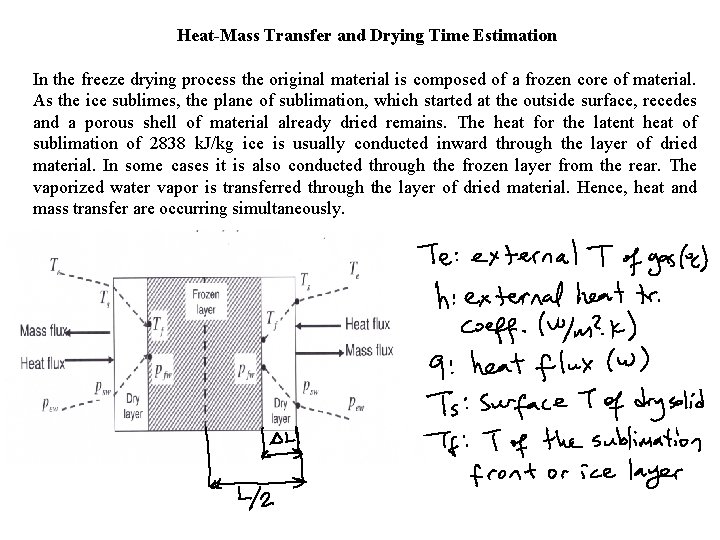
Heat-Mass Transfer and Drying Time Estimation In the freeze drying process the original material is composed of a frozen core of material. As the ice sublimes, the plane of sublimation, which started at the outside surface, recedes and a porous shell of material already dried remains. The heat for the latent heat of sublimation of 2838 k. J/kg ice is usually conducted inward through the layer of dried material. In some cases it is also conducted through the frozen layer from the rear. The vaporized water vapor is transferred through the layer of dried material. Hence, heat and mass transfer are occurring simultaneously.
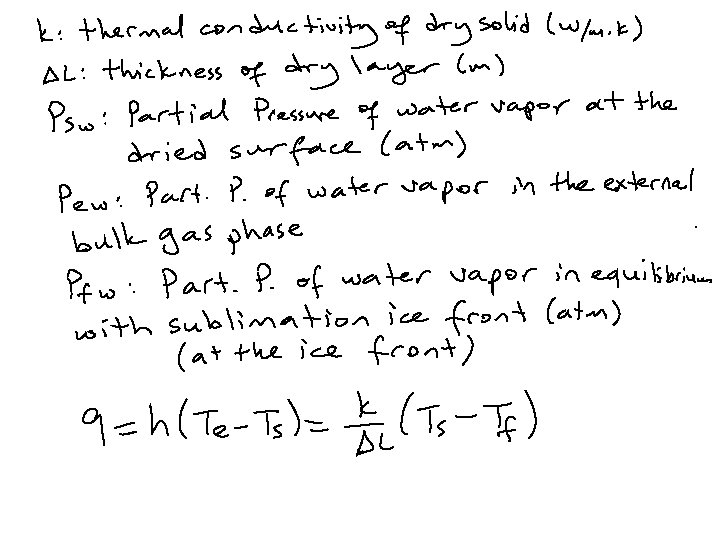

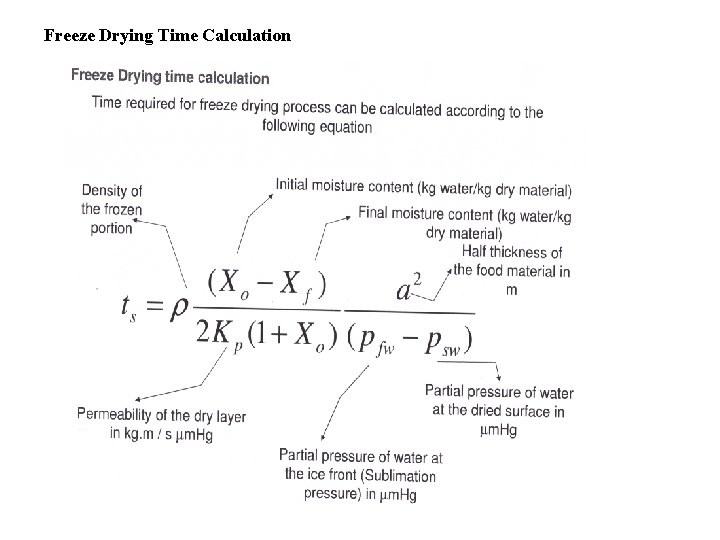
Freeze Drying Time Calculation

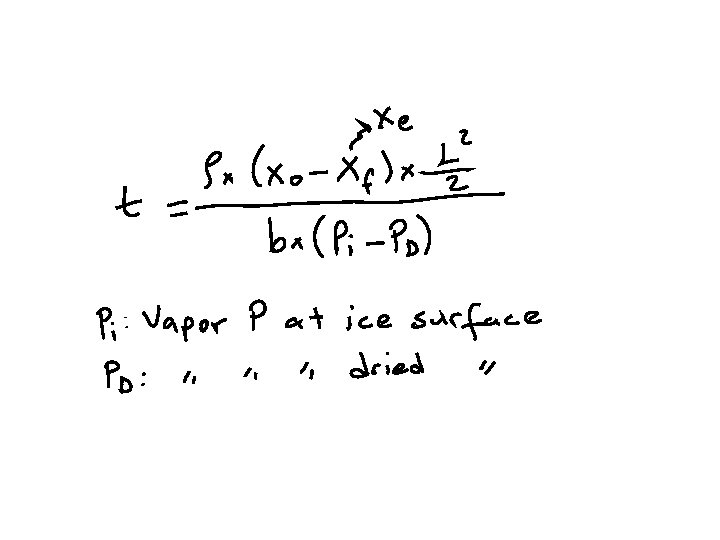

Consider a homogeneous slab of frozen food, at a temperature well below melting, undergoing lyophilization. It is desired to calculate the freeze drying time of the slab. The following assumptions are made: ● The slab is dried from one side only ● Heat is supplied to the surface of the slab by radiation from a hot surface at a distance from the slab ● All the heat supplied is used exclusively for the sublimation of ice crystals. As long as sublimation occurs, sensible heat effects are negligible ● There is a sharp sublimation front between the totally iceless (dry) zone and the frozen zone ● The vapors are condensed on a cold surface (condenser) as ice. The resistance of the chamber space between the slab surface and the condenser to mass transfer is negligible. Hence, the water vapor pressure at the condenser surface is equal to that measured in the chamber ● The temperature at the slab surface is kept constant, by controlling the temperature of the radiating body ● The temperature of the frozen product and hence of the sublimation front is constant ● The gas in the freeze dryer chamber consists, practically, of water vapor only. The proportion of non-condensables (air) is negligible.