Lecture 14 a Drying Solvents Conventional Drying Agents






- Slides: 9

Lecture 14 a Drying Solvents

Conventional Drying Agents • Usually drying agents like anhydrous Na 2 SO 4 or Mg. SO 4 are used to dry organic solutions • They remove the majority of the water but not all of it because the drying process is an equilibrium reaction • They adsorb varying amount of water (n=0. 5 moles (Ca. SO 4) to n=10 moles (Na 2 SO 4)) • Their efficiency is measured by intensity, capacity and velocity can greatly vary from one solvent to the other • Problem: The water is just absorbed by the drying agent and not “consumed”

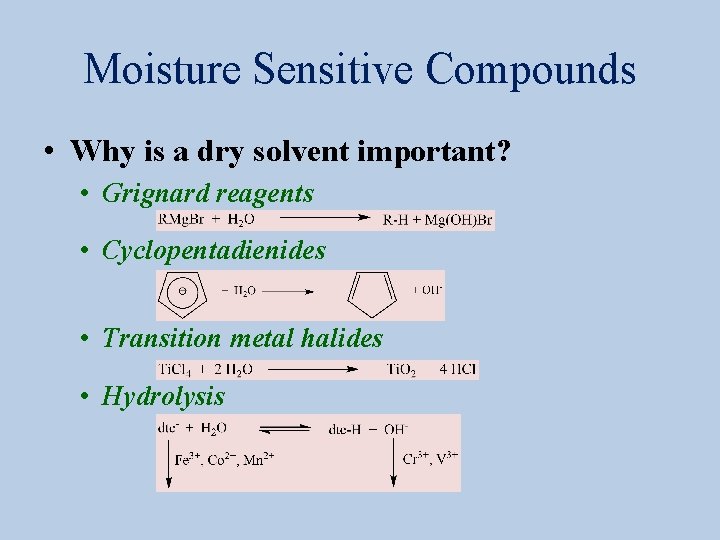
Moisture Sensitive Compounds • Why is a dry solvent important? • Grignard reagents • Cyclopentadienides • Transition metal halides • Hydrolysis

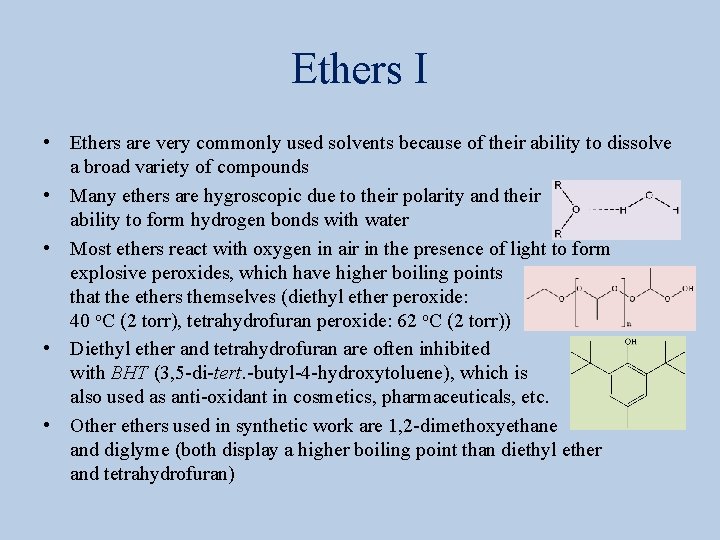
Ethers I • Ethers are very commonly used solvents because of their ability to dissolve a broad variety of compounds • Many ethers are hygroscopic due to their polarity and their ability to form hydrogen bonds with water • Most ethers react with oxygen in air in the presence of light to form explosive peroxides, which have higher boiling points that the ethers themselves (diethyl ether peroxide: 40 o. C (2 torr), tetrahydrofuran peroxide: 62 o. C (2 torr)) • Diethyl ether and tetrahydrofuran are often inhibited with BHT (3, 5 -di-tert. -butyl-4 -hydroxytoluene), which is also used as anti-oxidant in cosmetics, pharmaceuticals, etc. • Other ethers used in synthetic work are 1, 2 -dimethoxyethane and diglyme (both display a higher boiling point than diethyl ether and tetrahydrofuran)

Ethers II • Purification • Step 1: Test for peroxides with KI-starch paper (turns dark blue) or acidic KI-solution (turn yellow-brown) in the presence of peroxides • Step 2: Removal of water and peroxides by treatment with sodium/benzophenone (color change from beige to dark blue) • Due to the formation of hydrogen gas the reaction because irreversible • The dark blue color is due to a ketyl radical anion (Ph 2 CO. -Na+), which is only stable in the absence of oxidants and water • Alternatively Li. Al. H 4 or Ca. H 2 can be used as drying agents for less rigorous applications • This approach can also be used for many hydrocarbons i. e. , toluene

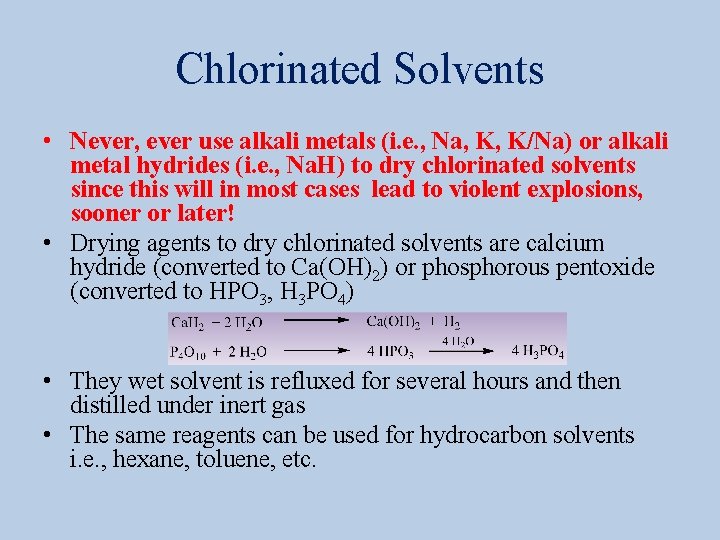
Chlorinated Solvents • Never, ever use alkali metals (i. e. , Na, K, K/Na) or alkali metal hydrides (i. e. , Na. H) to dry chlorinated solvents since this will in most cases lead to violent explosions, sooner or later! • Drying agents to dry chlorinated solvents are calcium hydride (converted to Ca(OH)2) or phosphorous pentoxide (converted to HPO 3, H 3 PO 4) • They wet solvent is refluxed for several hours and then distilled under inert gas • The same reagents can be used for hydrocarbon solvents i. e. , hexane, toluene, etc.

Other Solvents I • • • Water • Dissolved salts may be removed by distillation or ion exchange. • It can be degassed purging it with an inert gas for an extended time. • Alternatively, several freeze-pump-thaw cycles can help to remove dissolved gases (i. e. , oxygen). Alcohols • Alcohols are mainly contaminated with varying amounts of water • Ethanol: Ca. O or Na/diethyl phthalate • Methanol: fractionated distillation, Na/dimethyl phthalate N, N-Dimethylformamide • Dimethyl formamide (DMF) is contaminated by dimethylamine • Anhydrous magnesium sulfate is used to remove the majority of the water (final concentration: ~ 0. 01 M) followed by a vacuum distillation. • For higher quality, the pre-dried solvent can stored over Ba. O before it is distilled over alumina (50 g/L). • The pre-dried solvent can be refluxed with triphenylchlorosilane (Ph 3 Si. Cl) for 24 hours.

Other Solvents II • Dimethyl sulfoxide • Contaminated by water • Reflux over Ca. H 2 and then distillation in vacuo • Acetone • • Acetone is contaminated by aldehydes (i. e. , acetaldehyde), which can be removed by treatment with silver nitrate or potassium permanganate • For less rigorous applications, drying over anhydrous calcium sulfate or potassium carbonate provides good results • For more sensitive application, the pre-dried solvent can be refluxed over Ca. H 2 and afterwards over P 4 O 10 Acetonitrile • • • Acetonitrile is contaminated with acetamide, ammonia and ammonium acetate. Often times, it is pre-dried with calcium hydride and then refluxed over phosphorus pentoxide. If the pre-drying step is skipped, the formation of an orange polymer will be observed during the drying process.

Summary • Removal of water and other compounds is important to maintain the quality of the reagents, optimize yields and reduce undesirable side reactions • Obtaining very pure solvents can be an arduous task in some cases since the purification usually involves many steps and extended reflux in most cases • The purified solvents are often stored under inert gas and over a molecular sieve to keep them dry for some time (Note that the molecular sieve has to have the correct porosity (i. e. , 4 Å) and also has to be properly activated prior to its use!) • Maintaining the solvent purification systems is also very important to avoid unpleasant surprises i. e. , disintegrating flasks, explosion due to the build-up of peroxides, etc.