Fossils Evidence of Past Life Fossils Fossils are











- Slides: 16

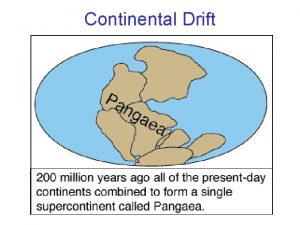
Fossils: Evidence of Past Life

Fossils • Fossils are the remains or traces of prehistoric life.

Fossils • Unaltered Remains • • Remains of organisms that haven’t changed, or have barely changed over time Name some examples or unaltered remains…

Fossils • Altered Remains • • • The remains of an organism are likely to be changed over time. Fossils can often become petrified (turned to stone) Molds and casts are also common fossils.

Fossils • Indirect Evidence • Trace fossils are indirect evidence of prehistoric life.

Fossils • Conditions Favoring Preservation • Two conditions are important for preservation: rapid burial and the possession of hard parts.


Dating with Radioactivity

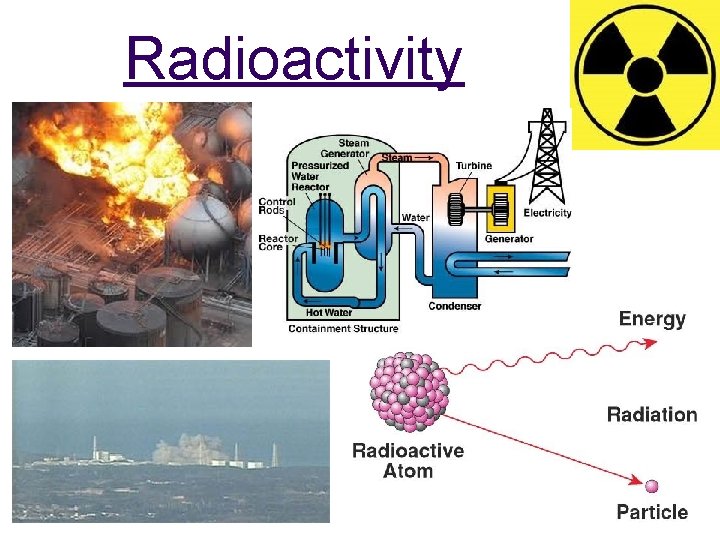
Dating with Radioactivity • What is radioactivity? Radioactivity is the spontaneous decay of certain unstable atomic nuclei.

Radioactivity

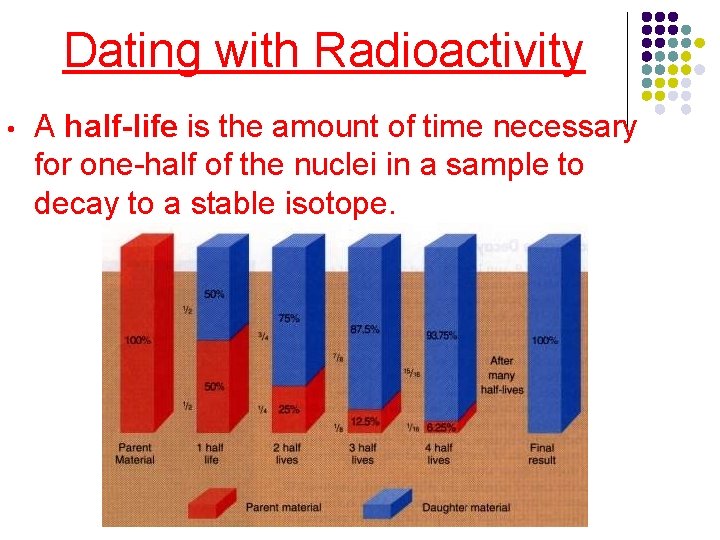
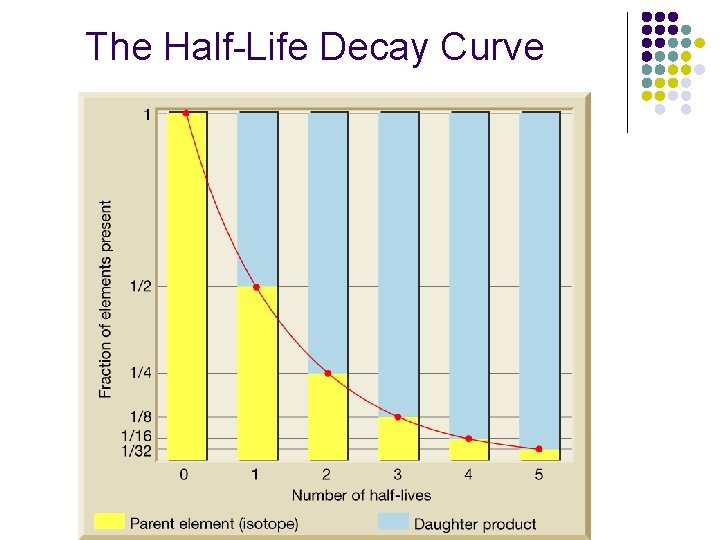
Dating with Radioactivity • A half-life is the amount of time necessary for one-half of the nuclei in a sample to decay to a stable isotope.

Dating with Radioactivity • Every isotope decayschanges at a constant rate • Radiometric dating is calculating a rocks age by examining the amount radioactive isotopes present

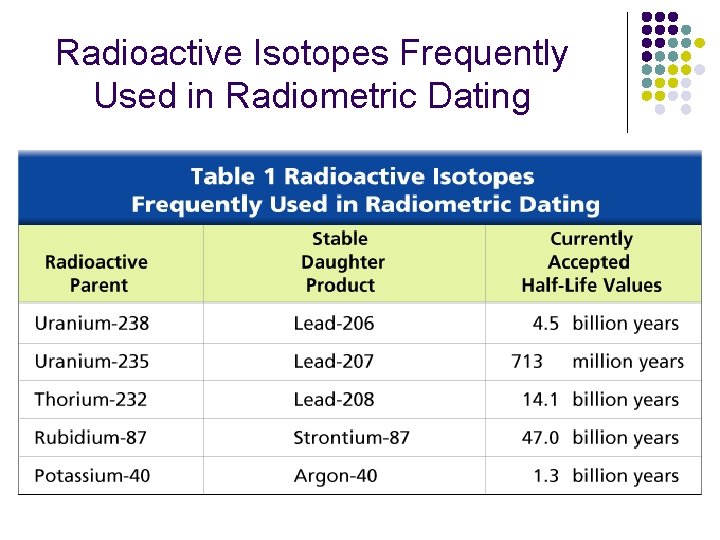
Dating with Radioactivity • Radioactive isotopes decay (or change) into atoms called daughter products • Examples: • Carbon-14 decays to Carbon-12 • 1 half-life = 5, 730 years • Unranium-235 decays to Lead-207 • 1 half-life = 713 million years • Urannium-238 decays to Lead-206 • 1 half-life = 4. 5 billion years • Radiometric dating only works if the fossil has remained in a closed system since it became a fossil

The Half-Life Decay Curve

Radioactive Isotopes Frequently Used in Radiometric Dating

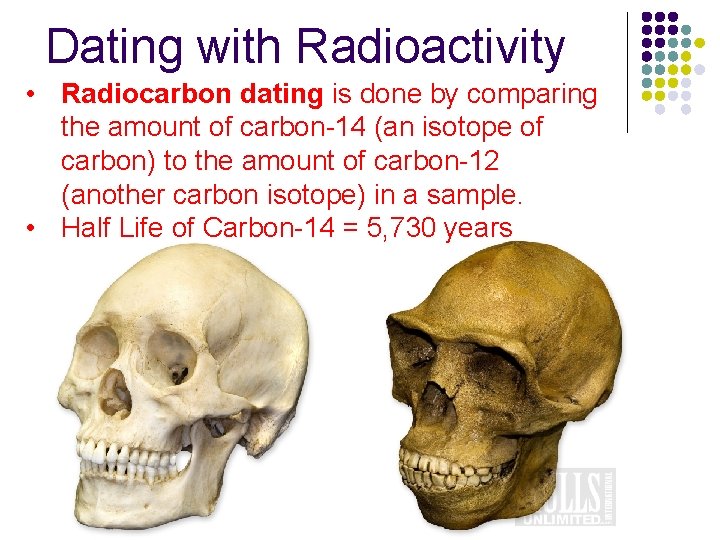
Dating with Radioactivity • Radiocarbon dating is done by comparing the amount of carbon-14 (an isotope of carbon) to the amount of carbon-12 (another carbon isotope) in a sample. • Half Life of Carbon-14 = 5, 730 years
 Insidan region jh
Insidan region jh Fossils evidence of past life
Fossils evidence of past life Glossopeteris
Glossopeteris What does amber
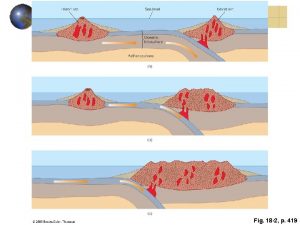
What does amber Chapter 10 plate tectonics
Chapter 10 plate tectonics Indirect fossil
Indirect fossil Secondary sources
Secondary sources Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Secondary sources
Secondary sources Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Fiber evidence can have probative value.
Fiber evidence can have probative value. Class vs individual evidence
Class vs individual evidence Individual evidence can have probative value
Individual evidence can have probative value Individual vs class evidence
Individual vs class evidence Genetic fallacy definition
Genetic fallacy definition What can fossils tell us
What can fossils tell us