Forensic Science Analysis of Glass and Soil Evidence












































- Slides: 56

Forensic Science: Analysis of Glass and Soil Evidence

Glass evidence can be found at many crime scenes. • Automobile accident sites may be littered with broken headlight or windshield glass. • The site of a store break-in may contain shards of window glass with fibers or blood on them. • If shots are fired into a window, the sequence and direction of the bullets can often be determined by examining the glass. • Minute particles of glass may be transferred to a suspect’s shoes or clothing and can provide a source of trace evidence linking a suspect to a crime.

Glass & Forensics • How was it broken? • Link a suspect to a crime scene • Fingerprints • Blood

Chemical and Physical Properties • Physical properties describe a substance without comparing with another substance. They are measurements like weight, volume, boiling point, melting point. • Chemical properties describe what happens when you combine it with something else in a chemical reaction. Examples are burning, coloring reagent tests, decomposing, synthesis of an alloy from individual elements

How is glass formed? • Long before humans began making glass, glass formed naturally. • When certain types of rock are exposed to extremely high temperatures, such as lightning strikes or erupting volcanoes, glass can form. • Obsidian is a type of glass formed by volcanoes.

Timeline of Events • Prehistoric humans used obsidian as a cutting tool. • The earliest man-made glass objects (glass beads) were found in Egypt dating back to 2500 BC. • Glass blowing began sometime during the first century BC. • By the 14 th century, knowledge of glass making spread throughout Europe. • The Industrial Revolution brought the mass production of many kinds of glass.

How is Glass Formed? • Glass is a hard, brittle, amorphous material made by melting sand (aka silica, silicon dioxide, Si. O 2) lime (aka calcium oxide Ca. O) and soda, sodium carbonate (Na 2 CO 3) at very high temperatures. • The lime (Ca. O) is added to prevent the glass from being soluble in water. • The soda (Na 2 CO 3) is added to lower the melting point of silica (sand) and make it easier to work with. • In some types of glass with special requirements, trace amounts of other elements are added. Example: Boron is added to make Pyrex glass.

How is Glass Made? • Following the mixing of the raw materials, they are transported to the furnace and heated to over 1200 o. C or 2200 o. F and changed into a molten mixture. • There are different formulas and assembly for different glass applications. Ex: car wind shields are 2 layers with plastic in between.

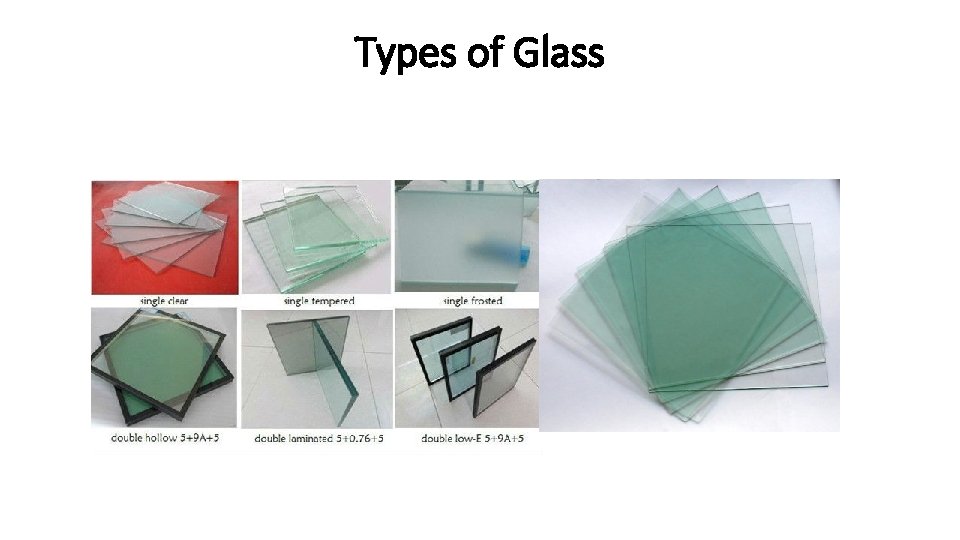
Types of Glass

Types of Glass – soda-lime glass • Mostly sand, sodium carbonate and calcium oxide: • Used for manufacturing most window and bottle glass • Making Window Panes • Making Glass Bottles • Rolling Glass

Types of Glass - Float Glass • Flat glass typically used for windows. • Soda-lime glass that has been cooled on top of a bath of molten tin.

Types of glass - Leaded Glass • Fine glassware and decorative art glass, called crystal or leaded glass substitutes lead oxide for calcium oxide (lime). • The addition of lead oxide makes the glass denser. As light passes through the more-dense glass, the light waves are bent, giving the glass a sparkling effect.

Types of glass - Tempered Glass • This glass is made stronger than ordinary window glass by introducing stress through rapid heating and cooling of the glass surfaces. • When tempered glass breaks, it does not shatter but rather fragments or “dices” into small squares with little splintering. • Used for side and rear windows of automobiles sold in the United States.

Types of glass - Bulletproof Glass • Bulletproof glass is a combination of two or more types of glass, one hard and one soft. • The softer layer makes the glass more elastic so it can flex instead of shatter. • The index of refraction for both of the glasses used in the bulletproof layers must be almost the same to keep the glass transparent and allow a clear view through the glass. • Bulletproof glass varies in thickness from three-quarter inch to three inches.

Density of Glass Determination • To determine the density of glass, it is best to use the immersion method. • Density = mass/volume • Mass is measured on a balance / scale. • Volume is determined by immersing parts of the glass and seeing the level change in a measurement glassware.

Comparing Densities: Flotation • A solid particle will either float, sink, or remain suspended in a liquid, depending upon its density relative to the liquid medium. • Flotation = a standard / reference glass particle is immersed in a liquid; a mixture of bromoform or bromobenzene may be used. The composition of the liquid is carefully adjusted by adding small amounts of bromoform or bromobenzene until the glass chip remains suspended in the liquid medium. At this point, the standard / reference glass sample and the liquid each have the same density. Glass chips (same size and shape as reference sample) are added to the liquid for comparison. If both the unknown and standard / reference samples remain suspended, they have the same density.

Flotation A standard / reference glass particle is immersed in a liquid; a mixture of bromoform or bromobenzene may be used. The composition of the liquid is carefully adjusted by adding small amounts of bromoform or bromobenzene until the glass chip remains suspended in the liquid medium. At this point, the standard / reference glass sample and the liquid each have the same density. Glass chips (same size and shape as reference sample) are added to the liquid for comparison. If both the unknown and standard / reference samples remain suspended, they have the same density.

Refraction of Light • Refraction of light is the bending of the light as it passes the boundary between two different optically dense mediums. It changes directions because it changes speed

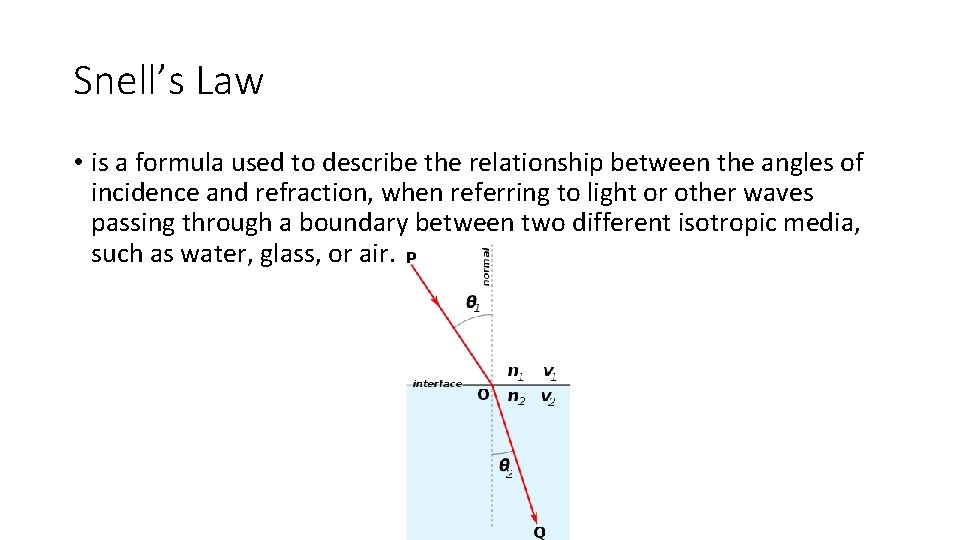
Snell’s Law • is a formula used to describe the relationship between the angles of incidence and refraction, when referring to light or other waves passing through a boundary between two different isotropic media, such as water, glass, or air.

Refractive Index • When light changes from space to air to water, it slows down. This changes the path it takes. That’s refraction. • The refractive index is the ratio of the velocity of light in space to the velocity inside a different material. • Refractive index = Velocity in space • Velocity in medium • Think of it as the optical density of a material. The thicker it is the slower you go

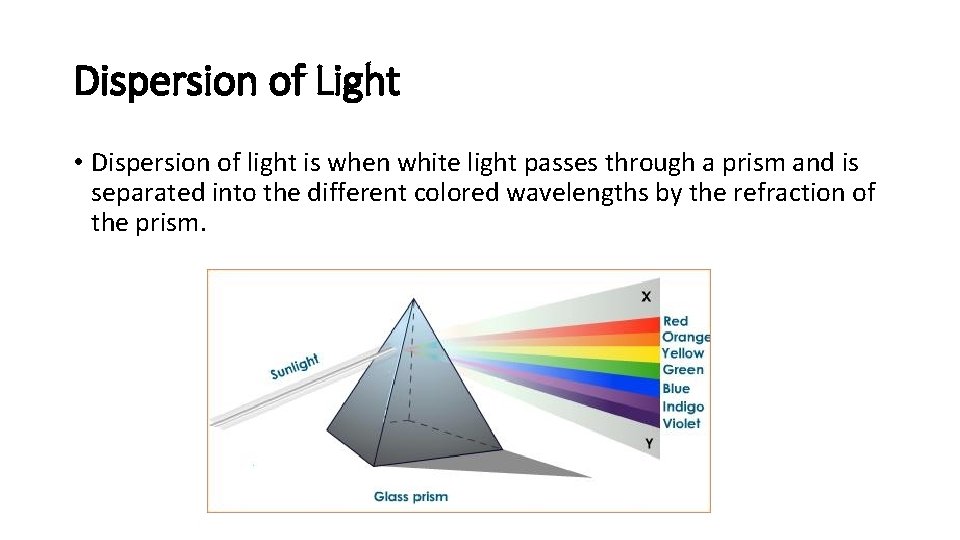
Dispersion of Light • Dispersion of light is when white light passes through a prism and is separated into the different colored wavelengths by the refraction of the prism.

Forensics of Broken Glass • When broken glass is found at a crime scene it is gathered and checked for several things: • • Puzzle pieces fitting together. Fracture pattern. Blood or any DNA source Fingerprints Composition or type of glass Density – mass per unit of volume. Determining refractive index. Any unique characteristics: ex. Paint, scratches

Proper Collection of Glass Evidence • Standard reference glass should be taken from the crime scene (1 in 2) • Package in solid containers to prevent breakage • Preserve garment (shoe, pants, shirt) with glass on it • All broken glass must be recovered and submitted for analysis when direction of impact is desired. • Whenever possible, the exterior and interior surfaces of the glass must be indicated. The presence of dirt, paint, grease or putty may indicate the exterior surface of the glass.

Jigsaw Effect – Most Beneficial • When the suspect and crime-scene fragments are assembled and physically fitted together. • Comparisons of this type require piecing together irregular edges of broken glass as well as matching all irregularities and striations on the broken surfaces. The possibility that two pieces of glass originating from different sources will fit together exactly is so unlikely as to exclude all other sources from practical consideration. • Unfortunately, most glass evidence is either too fragmentary or too minute to permit a comparison of this type

Soil Analysis “Life is hard. Then you die. Then they throw dirt in your face. Then the worms eat you. Be grateful it happens in that order. ” —David Gerrold, American science fiction writer

Soil Analysis Students will learn to: § Identify a soil’s common constituents § Determine the origin of a soil sample § Why soils can be used as class evidence § When soils can be used as circumstantial evidence 26

Forensic Geology § The legal application of earth and soil science § Characterization of earthen materials that have been transferred between objects or locations and the analysis of possible origin or sources 27

Soil A. Definition—naturally deposited materials that cover the earth’s surface and are capable of supporting plant growth B. The Earth 75%—oceans, seas and lakes 15%—deserts, polar ice caps and mountains 10%—suitable for agriculture 28

Soil C. Formation § § § 29 Living matter—plants, animals, microorganisms Inorganic materials Climate Parent materials Relief—slope and land form Time

Soil D. Profile § Topsoil § Subsoil § Parent material 30 E. Composition § Sand § Silt § Clay § Organic matter

Soil F. Nutrients—macro § Nitrogen § Phosphorus § Potassium § Calcium § Magnesium § Sulfur 31 G. Nutrients—micro § § § § Manganese Iron Boron Copper Zinc Molybdenum Chlorine

Soil Comparisons § May establish a relationship or link to the crime, the victim, or the suspect(s) § Physical properties—density, magnetism, particle size, mineralogy § Chemical properties—p. H, trace elements 32

Probative Value of Soil § Types of earth material are virtually unlimited. They have a wide distribution and change over short distances. § As a result, the statistical probability of a given sample having properties the same as another is very small § Evidential value of soil can be excellent 33

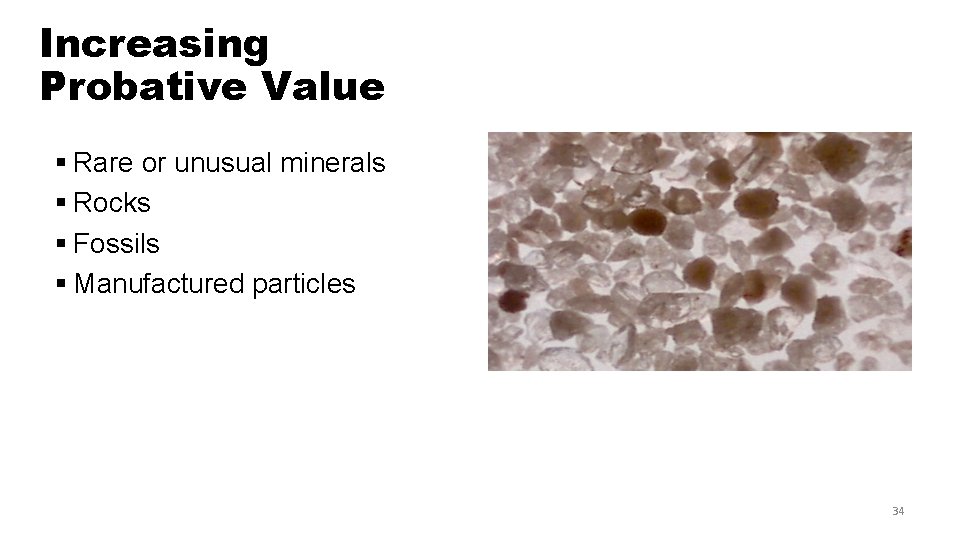
Increasing Probative Value § Rare or unusual minerals § Rocks § Fossils § Manufactured particles 34

Minerals § More than 2000 have been identified § Twenty or so are commonly found in soils; most soil samples contain only 3 to 5 § Characteristics for identification—size, density, color, luster, fracture, streak, or magnetism 35

Rocks § Aggregates of minerals § Types § Natural—like granite § Man-made—like concrete § Formation § Igneous § Sedimentary § Metamorphic 36

Palynology § The study of pollen and spores § Important to know: § What is produced in a given area § The dispersal pattern § Variation in size and weight 37

EQ: How is soil analyzed in forensic science?

Forensic Definition of Soil • Any disintegrated surface material, natural or artificial that lies on or near the Earth’s surface. • Natural= rocks, minerals, vegetation, animal matter • Manufactured= glass, paint, asphalt, brick fragments, cinders

Soil • The value of soil as evidence rests with its prevalence at crime scenes and its transferability between the scene and the criminal. • Most soils can be differentiated by their appearance and color. • The first step in examination of soil is a side-byside examination of color and texture.


Soil Types-Texture • People describe soil types in all kinds of ways such as heavy, light, sandy, clay, loam, poor or good. • Soil scientists describe soil types by how much sand, silt and clay are present. This is called texture.

What is the first step in the examination of soil? Side-by-side comparison of color and texture

Comparison Microscope • Soil appears different when wet, therefore samples are dried in the same manner in the lab • 1, 100 distinguishable soil colors • Low power magnification offers presence of plant and animal debris • High magnification can classify minerals and rocks
Mineral • Naturally occurring crystalline solid • UNIQUE • • COLOR GEOMETRIC SHAPE DENSITY REFRACTIVE INDEX • 2200 exist, but only 20 are common and found readily at the surface

Rocks • Made of a combination of minerals • Characterized by their mineral content and grain size

Mineral Analysis • Rocks and minerals are used to manufacture a wide variety of industrial and commercial products; safe insulation, brick, plaster and concrete blocks for example.

Which magnification allows you to see naturally occuring crystalline solids? High Magnification

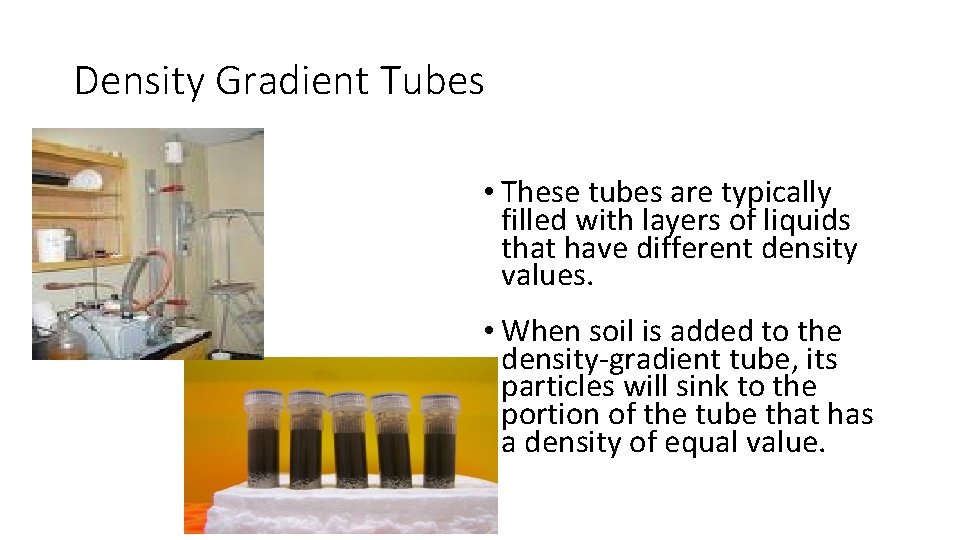
Density Gradient Tubes • These tubes are typically filled with layers of liquids that have different density values. • When soil is added to the density-gradient tube, its particles will sink to the portion of the tube that has a density of equal value.

What is the main issue with the use of Density Gradient tubes? • Many soils from different areas yield similar densities

Variations in Soil Crime Scene • Ultimate value depends on its variation at the crime scene • If the soil is indistinguishable for miles surrounding the crime scene, it will have limited value in associating soil found on the suspect with that particular site. • Variations in soil composition must be made every 10 -100 yards from the crime site.

Collection of Soil • Standard/reference soils are to be collected at various intervals within a 100 -yard radius of the crime scene, as well as the site of the crime, for comparison to the questioned soil. • Soil found on the suspect, such as adhering to a shoe or garments, must not be removed. • Instead, each object should be individually wrapped in paper, and stored in either plastic jars or ziplock plastic bags and transmitted to the laboratory.

Collection • Soil must be collected at all alibi locations that the suspect claims • For Standard/ Reference samples: In most cases, only a tablespoon or two of the top layer of soil is collected, placed in individual plastic containers, and labeled according to location.

Where all the locations soil should be collected? Crime scene, 100 yds radius of crime scene, alibi locations, any object or clothing of interest that contains soil


 Glass evidence in forensic science
Glass evidence in forensic science Flotation method glass
Flotation method glass Radial fracture glass
Radial fracture glass What is transient evidence
What is transient evidence Testimonial evidence examples
Testimonial evidence examples Forensic pathologist vs forensic anthropologist
Forensic pathologist vs forensic anthropologist Forensic psychiatry vs forensic psychology
Forensic psychiatry vs forensic psychology Fibers from animals
Fibers from animals The forensic definition of soil is
The forensic definition of soil is Living soil vs dead soil
Living soil vs dead soil Living soil vs dead soil
Living soil vs dead soil Forensic recovery of evidence device
Forensic recovery of evidence device How to maintain a qualified forensic duplicate
How to maintain a qualified forensic duplicate Kathleen peterson forensic evidence
Kathleen peterson forensic evidence Forensic evidence
Forensic evidence Tire track width
Tire track width Glass escalator concept
Glass escalator concept If acid is splashed on your skin wash at once with
If acid is splashed on your skin wash at once with Does hot glass look like cold glass
Does hot glass look like cold glass Difference between class and individual evidence
Difference between class and individual evidence Trace evidence soil
Trace evidence soil Hard soil evidence
Hard soil evidence Hard soil evidence
Hard soil evidence What s your favorite subject
What s your favorite subject Forensic science fundamentals and investigations chapter 6
Forensic science fundamentals and investigations chapter 6 Chapter 4 fibers and textiles
Chapter 4 fibers and textiles Chapter 1 intro to forensic science
Chapter 1 intro to forensic science Hairs and fibers in forensic science
Hairs and fibers in forensic science Is glass class evidence
Is glass class evidence Advantages of fertilizers
Advantages of fertilizers What is a primary source
What is a primary source Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Secondary sources
Secondary sources Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Fiber evidence can have probative value as class evidence.
Fiber evidence can have probative value as class evidence. Class evidence vs individual evidence
Class evidence vs individual evidence Class vs individual evidence
Class vs individual evidence Red herring fallacy
Red herring fallacy Solapith hymen
Solapith hymen Morphology forensics
Morphology forensics Mass spectrometry in forensic science
Mass spectrometry in forensic science Branches of forensics
Branches of forensics Ted bundy signature behaviour
Ted bundy signature behaviour Forensic science history timeline
Forensic science history timeline Serology forensic science
Serology forensic science Unit 1 introduction to forensic science
Unit 1 introduction to forensic science Forensic science foodborne outbreak investigation answers
Forensic science foodborne outbreak investigation answers What is forensic science
What is forensic science Forensic science begins at the crime scene.
Forensic science begins at the crime scene. Forensic science arson activity
Forensic science arson activity Forensic science chapter 18 review answers
Forensic science chapter 18 review answers Observation forensic science definition
Observation forensic science definition Forensic science chapter 17 review answers
Forensic science chapter 17 review answers Chapter 14 review forensic science
Chapter 14 review forensic science Branches of forensic psychology
Branches of forensic psychology Ultraviolet photography in forensic
Ultraviolet photography in forensic