Fluorescence and Chemiluminescence Richard Vytek 2008 Luminescence Emission







- Slides: 18

Fluorescence and Chemiluminescence Richard Vytášek 2008

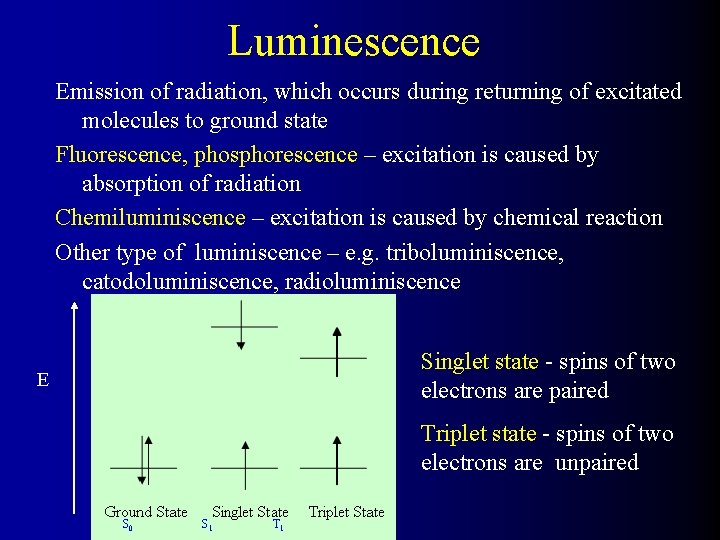
Luminescence Emission of radiation, which occurs during returning of excitated molecules to ground state Fluorescence, phosphorescence – excitation is caused by absorption of radiation Chemiluminiscence – excitation is caused by chemical reaction Other type of luminiscence – e. g. triboluminiscence, catodoluminiscence, radioluminiscence Singlet state - spins of two electrons are paired E Triplet state - spins of two electrons are unpaired S 0 S 1 T 1

Fluorescence and fosforescence

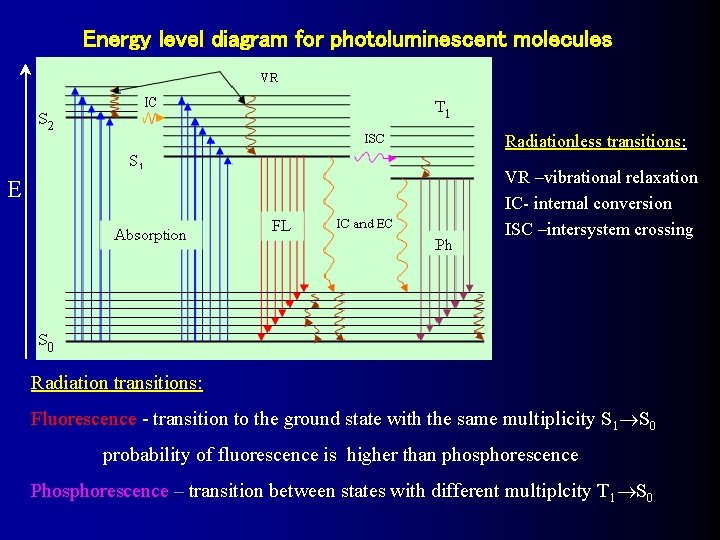
Energy level diagram for photoluminescent molecules Radiationless transitions: VR –vibrational relaxation IC- internal conversion ISC –intersystem crossing E Radiation transitions: Fluorescence - transition to the ground state with the same multiplicity S 1 S 0 probability of fluorescence is higher than phosphorescence Phosphorescence – transition between states with different multiplcity T 1 S 0

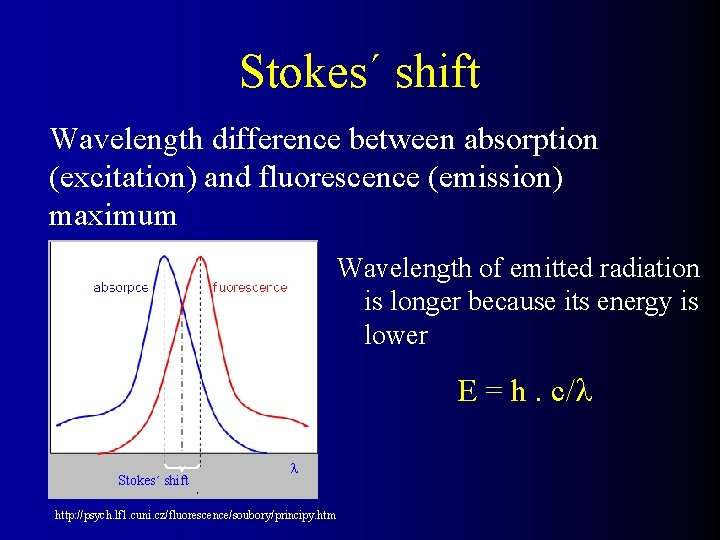
Stokes´ shift Wavelength difference between absorption (excitation) and fluorescence (emission) maximum Wavelength of emitted radiation is longer because its energy is lower E = h. c/ Stokes´ shift http: //psych. lf 1. cuni. cz/fluorescence/soubory/principy. htm

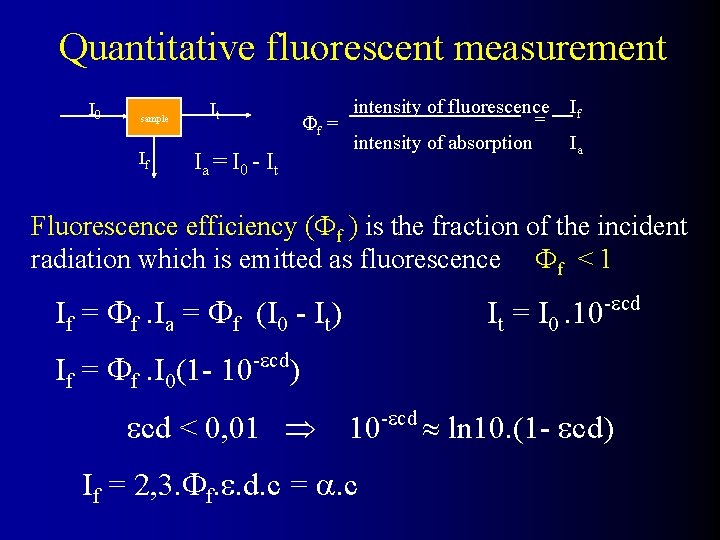
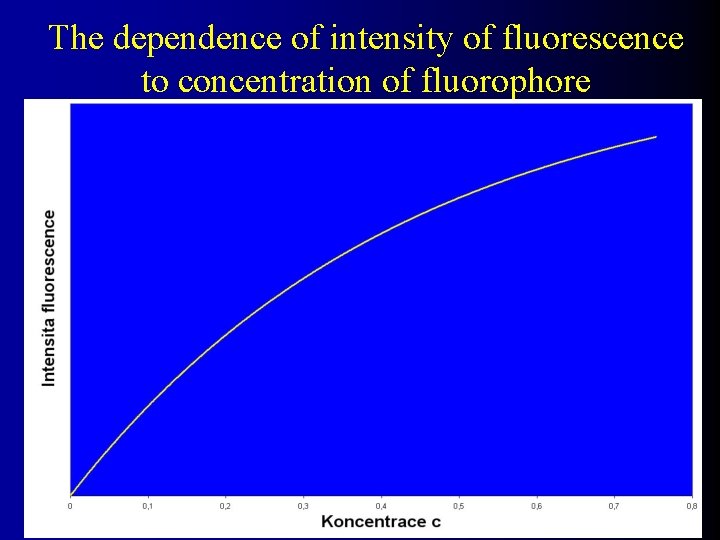
Quantitative fluorescent measurement I 0 sample If It f = Ia = I 0 - I t intensity of fluorescence If intensity of absorption Ia = Fluorescence efficiency ( f ) is the fraction of the incident radiation which is emitted as fluorescence f < 1 If = f. Ia = f (I 0 - It) If = f. I 0(1 - 10 -ecd It = I 0. 10 -ecd ) ecd < 0, 01 10 -ecd ln 10. (1 - ecd) If = 2, 3. f. e. d. c = . c

The dependence of intensity of fluorescence to concentration of fluorophore

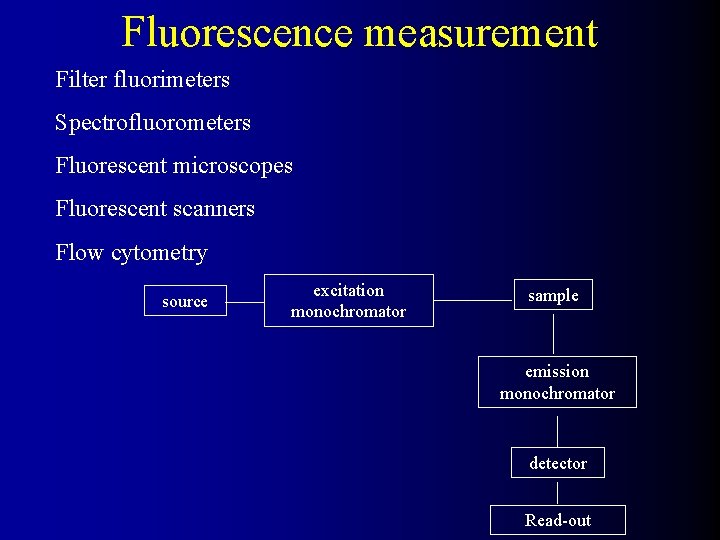
Fluorescence measurement Filter fluorimeters Spectrofluorometers Fluorescent microscopes Fluorescent scanners Flow cytometry source excitation monochromator sample emission monochromator detector Read-out

Sources of interference Inner filter effect intensity of excitation light isn´t constant because each layer of the sample absorbs some of the incident radiation (intensity of exciting light is higher in the front part of cuvette and lower in the rear part of cuvette Quenching excited molecule returns to the ground state by radiationless transition (without emitting light) as a result of a collision with quenching molecule Quenching agents: O 2, halogens (Br, I), nitrocompounds

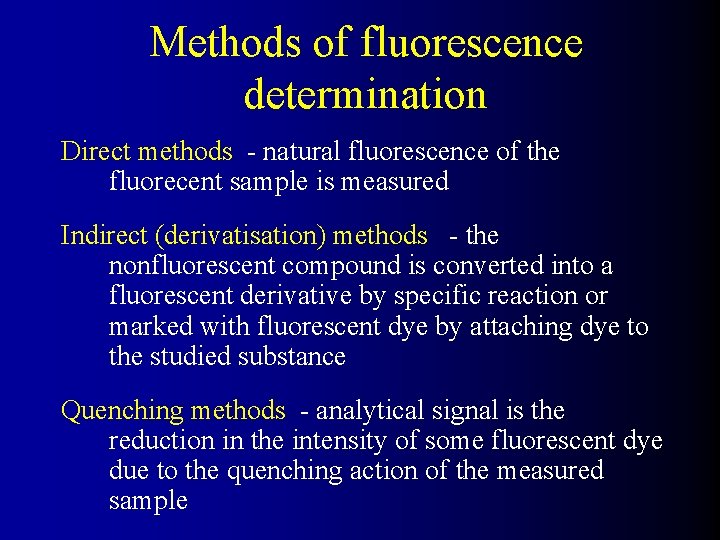
Methods of fluorescence determination Direct methods - natural fluorescence of the fluorecent sample is measured Indirect (derivatisation) methods - the nonfluorescent compound is converted into a fluorescent derivative by specific reaction or marked with fluorescent dye by attaching dye to the studied substance Quenching methods - analytical signal is the reduction in the intensity of some fluorescent dye due to the quenching action of the measured sample

Natural fluorophores • • • Lanthanides Polyaromatic hydrocarbons Vitamin A, E Coenzymes (FAD, FMN, NADH) Carotenes Quinine Steroids Aromatic aminoacids Nucleotides Fluorescent proteins –GFP (green fluorescent protein)

Nobel prize in chemistry in 2008 Osamu Shimomura discovered green fluorescent protein (GFP) in the small glowing jellyfish Aequorea victoria Martin Chalfie introduced using of green fluorescent protein as a marker for gene expression Roger Y. Tsien engineered different mutants of GFP with new optical properties (increased fluorescence, photostability and a shift of the major excitation peak ) and contributed to the explanation of mechanismus of GFP fluorescence

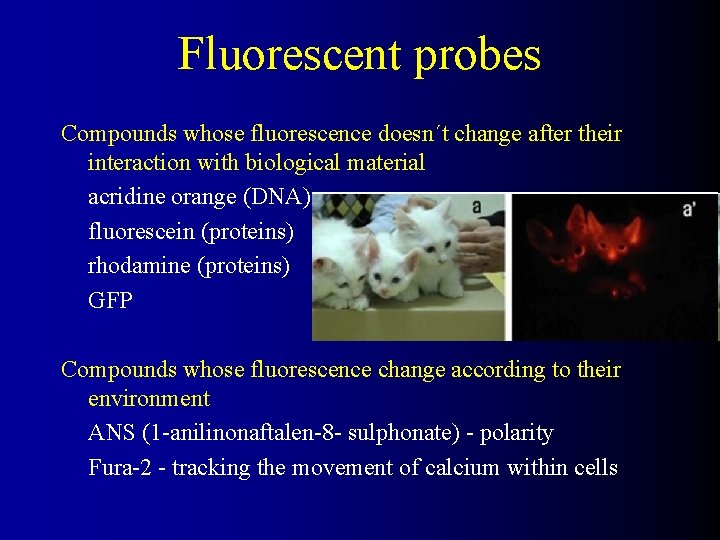
Fluorescent probes Compounds whose fluorescence doesn´t change after their interaction with biological material acridine orange (DNA) fluorescein (proteins) rhodamine (proteins) GFP Compounds whose fluorescence change according to their environment ANS (1 -anilinonaftalen-8 - sulphonate) - polarity Fura-2 - tracking the movement of calcium within cells

Some applications of fluorescence detection • • • Protein conformation Membrane potential Membrane transport Membrane viscosity Enzymatic reactions DNA analysis Genetic engineering (manipulations) Immunochemical methods Cell proliferation and apoptosis

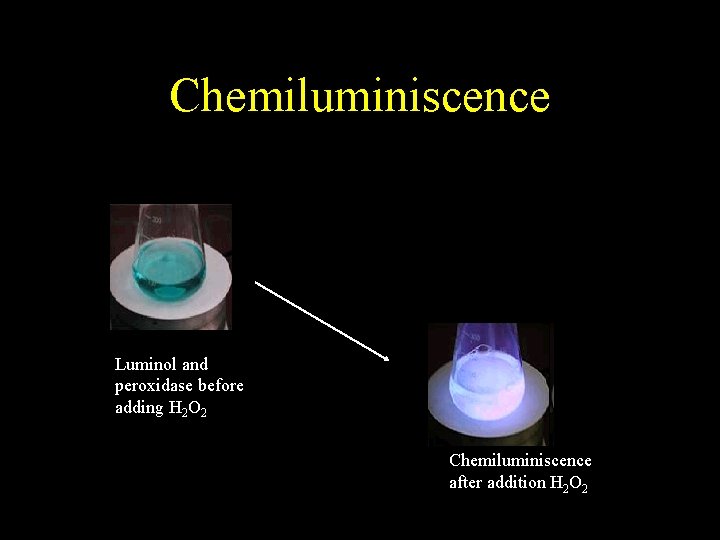
Chemiluminiscence Luminol and peroxidase before adding H 2 O 2 Chemiluminiscence after addition H 2 O 2

Chemiluminescence • Excitation of electrons is caused by chemical reaction • Return to ground state is accompanied by light emission Bioluminescence firefly Noctiluca scintillans ATP + luciferin + O 2 luciferase AMP + PPi + CO 2 + H 2 O + oxyluciferin + light

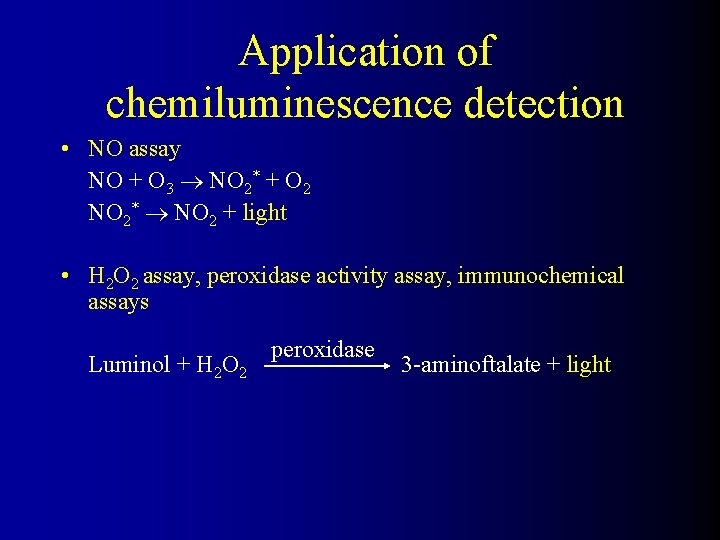
Application of chemiluminescence detection • NO assay NO + O 3 NO 2* + O 2 NO 2* NO 2 + light • H 2 O 2 assay, peroxidase activity assay, immunochemical assays Luminol + H 2 O 2 peroxidase 3 -aminoftalate + light

Thank you for your attention
 Chemiluminescence vs fluorescence
Chemiluminescence vs fluorescence Outline spectra
Outline spectra 2008 2008
2008 2008 King richard iii and looking for richard
King richard iii and looking for richard Beer's law fluorescence
Beer's law fluorescence Rfu relative fluorescence units
Rfu relative fluorescence units Fluorescence microscope uses
Fluorescence microscope uses Jablonski diagram
Jablonski diagram Principle of fluorescence spectroscopy
Principle of fluorescence spectroscopy Confocal fluorescence microscopy
Confocal fluorescence microscopy Bright field microscope specimen
Bright field microscope specimen Cold vapor atomic fluorescence spectrometry
Cold vapor atomic fluorescence spectrometry Light sources for fluorescence microscopy
Light sources for fluorescence microscopy Cold vapor atomic fluorescence spectrometry
Cold vapor atomic fluorescence spectrometry Atomic emission spectroscopy ppt
Atomic emission spectroscopy ppt Fluorescence activated cell sorting
Fluorescence activated cell sorting Fluorescence activated cell sorting
Fluorescence activated cell sorting Fluorescence bandpass filter
Fluorescence bandpass filter Atomic fluorescence spectroscopy principle
Atomic fluorescence spectroscopy principle