Flipping the Classroom Developing inclass Activities Focused on















- Slides: 20

Flipping the Classroom Developing in-class Activities Focused on High-level Cognitive Skills Freshman/Sophomore Level Course Junior/Senior Level Course

Flipping the classroom provides a dynamic and interactive learning environment where the educator guides students as they apply concepts and engage creatively in the subject matter Common concerns of flipping the classroom: • My students will not read the book or watch the video before lecture. • My students would rather listen to me lecture. • It sounds difficult. What kind of in-class activities do I use in my lower and upper level classes?


LECTURE (25 min) ACTIVITY (50 min)

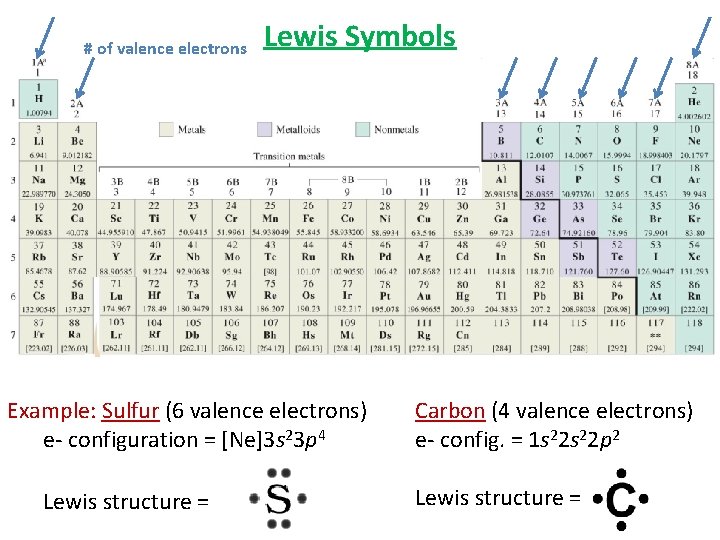
Lewis Symbols Electrons are shared or transferred between atoms in chemical bonds. Valence Electrons – the electrons involved in bonding. The outer shell electrons. Lewis Symbols • G. N. Lewis developed a simple way to show valence electrons of an atom • Consists of the element’s chemical symbol plus a dot ( • ) for each valence electron. Example: Sulfur (6 valence electrons) Carbon (4 valence electrons) e- configuration = [Ne]3 s 23 p 4 e- config. = 1 s 22 p 2 Lewis structure =

# of valence electrons Lewis Symbols Example: Sulfur (6 valence electrons) e- configuration = [Ne]3 s 23 p 4 Lewis structure = Carbon (4 valence electrons) e- config. = 1 s 22 p 2 Lewis structure =

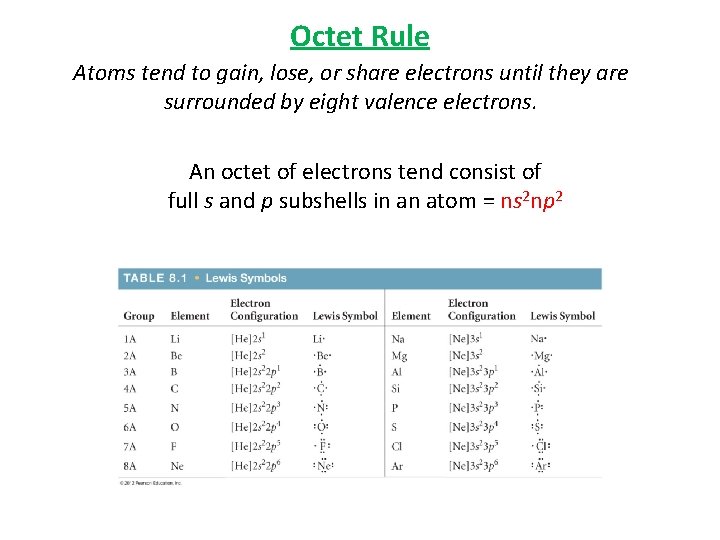
Octet Rule Atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons. An octet of electrons tend consist of full s and p subshells in an atom = ns 2 np 2

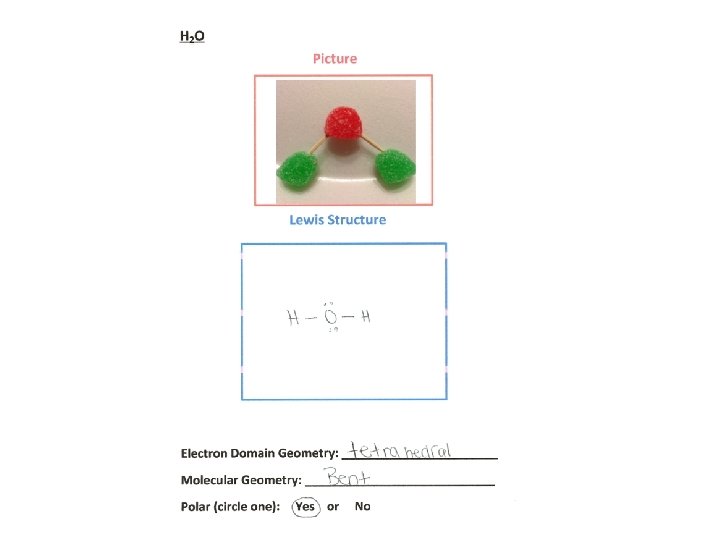
Lewis Structures • Formation of covalent bonds can be depicted with Lewis structures • These depict each electron pair shared between atoms as a line and show unshared electron pairs as dots. • Each pair of shared electrons constitutes one chemical bond.

Drawing Lewis Structures Practice, practice, and practice Follow the procedure: 1. Sum the valence electrons from all the atoms. Remember the periodic table can help you determine the number of valence electrons in each atom. For an anion, add one electron to the total for each negative charge. For a cation, subtract one electron from the total for each positive charge. 2. Write the symbols for the atoms, show which atoms are attached to which, and connect them with a single bond. Chemical formulas are often written in the order which the atoms are connected in a molecule or ion. For polyatomic ions and molecules, the central atom is usually written first. 3. Complete the octets around all the atoms bonded to the central atom. 4. Place any leftover electrons on the central atom. Do this even if so results in more than an octet of electrons around the atom. 5. If there are not enough electrons to give the central atom an octet, try multiple bonds. Use one or more of the unshared pairs of electrons on the atoms bonded to the central atom to form double or triple bonds.








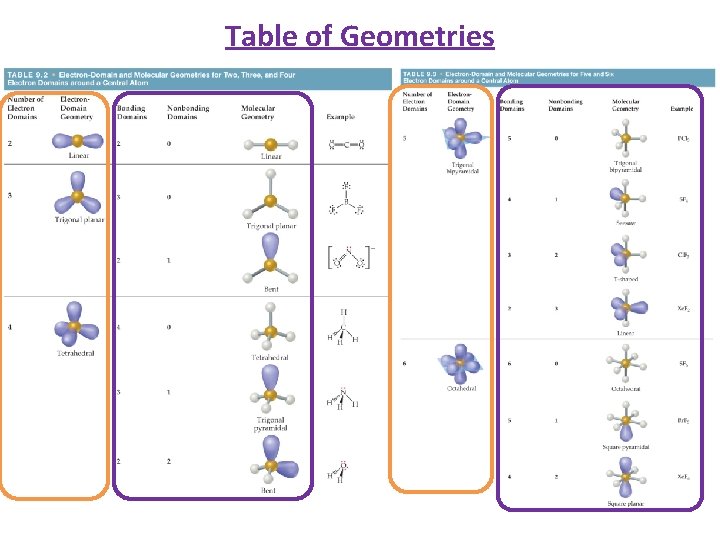
Table of Geometries


Thank You!
