EUROPEAN JOINT PROGRAMME ON RARE DISEASES EJP RD








- Slides: 15

EUROPEAN JOINT PROGRAMME ON RARE DISEASES (EJP RD)

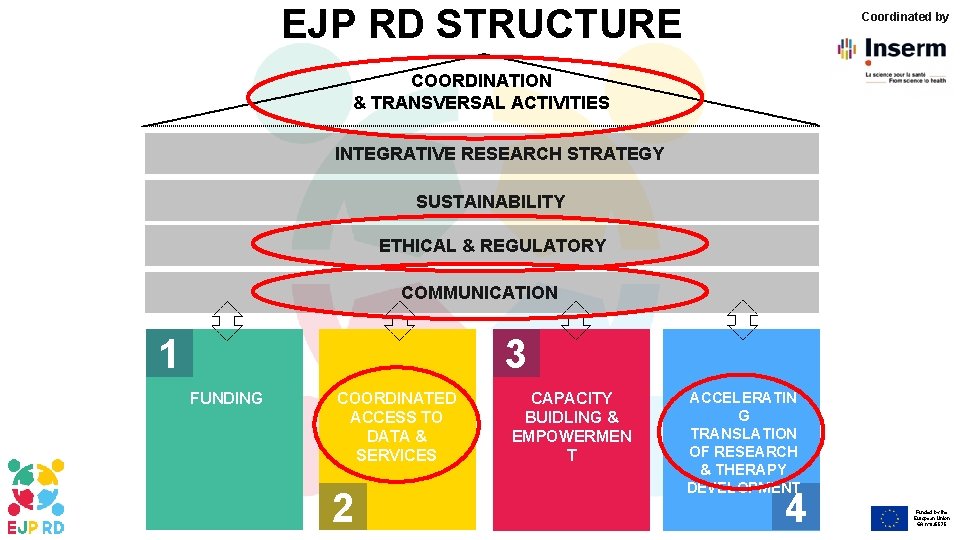
EJP RD STRUCTURE Coordinated by COORDINATION & TRANSVERSAL ACTIVITIES INTEGRATIVE RESEARCH STRATEGY SUSTAINABILITY ETHICAL & REGULATORY COMMUNICATION 3 1 FUNDING COORDINATED ACCESS TO DATA & SERVICES 2 CAPACITY BUIDLING & EMPOWERMEN T ACCELERATIN G TRANSLATION OF RESEARCH & THERAPY DEVELOPMENT 4 Funded by the European Union GA n° 825575

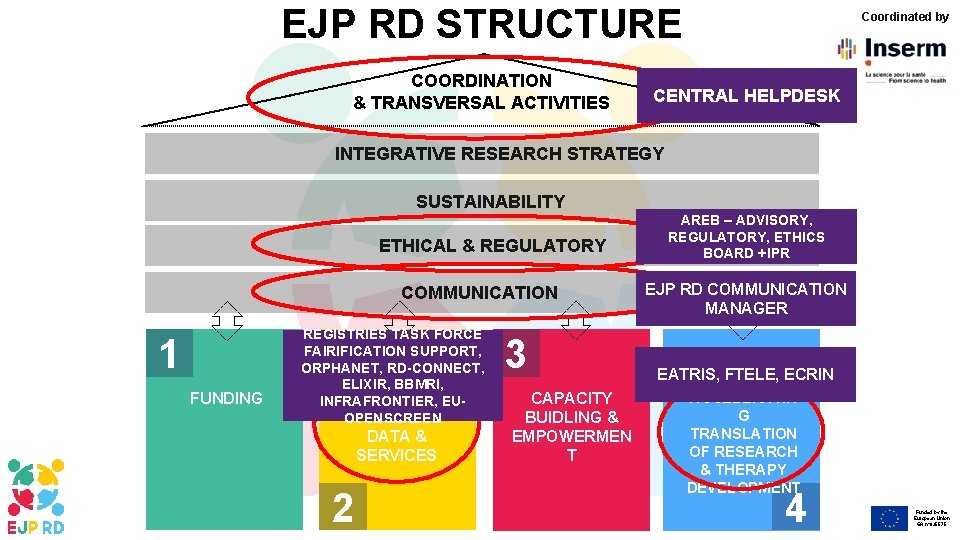
EJP RD STRUCTURE COORDINATION & TRANSVERSAL ACTIVITIES Coordinated by CENTRAL HELPDESK INTEGRATIVE RESEARCH STRATEGY SUSTAINABILITY ETHICAL & REGULATORY COMMUNICATION 1 FUNDING REGISTRIES TASK FORCE FAIRIFICATION SUPPORT, ORPHANET, RD-CONNECT, ELIXIR, BBMRI, COORDINATED INFRAFRONTIER, EUACCESS TO OPENSCREEN DATA & SERVICES 2 3 CAPACITY BUIDLING & EMPOWERMEN T AREB – ADVISORY, REGULATORY, ETHICS BOARD +IPR EJP RD COMMUNICATION MANAGER EATRIS, FTELE, ECRIN ACCELERATIN G TRANSLATION OF RESEARCH & THERAPY DEVELOPMENT 4 Funded by the European Union GA n° 825575

Central & transversal support Central Helpdesk: The Helpdesk is the single entry point for any inquiry related to the actions and services proposed within the EJP RD. It is managed by EJP RD project manager and takes advantage of the directory of external and internal experts and services that have been established for EJP RD. Mode of work Demands are classified according to a specific key: KEY 1: Question relative to EJP RD structure, operating, etc. KEY 2. 1: Question relative to EJP RD Pillar 1 – Collaborative research funding KEY 2. 2: Question relative to EJP RD Pillar 2 – Innovative coordinated access to data and services for transformative rare disease research KEY 2. 3: Question relative to EJP RD Pillar 3 – Capacity building and empowerment KEY 2. 4: Question relative to EJP RD Pillar 4 – Accelerating the translation of high potential projects and improving outcomes of clinical studies in small populations KEY 3. 1: Question requesting inter-pillar discussion KEY 3. 2: Question relative to any rare diseases topic outside of EJP RD activities and services Funded by the European Union GA n° 825575

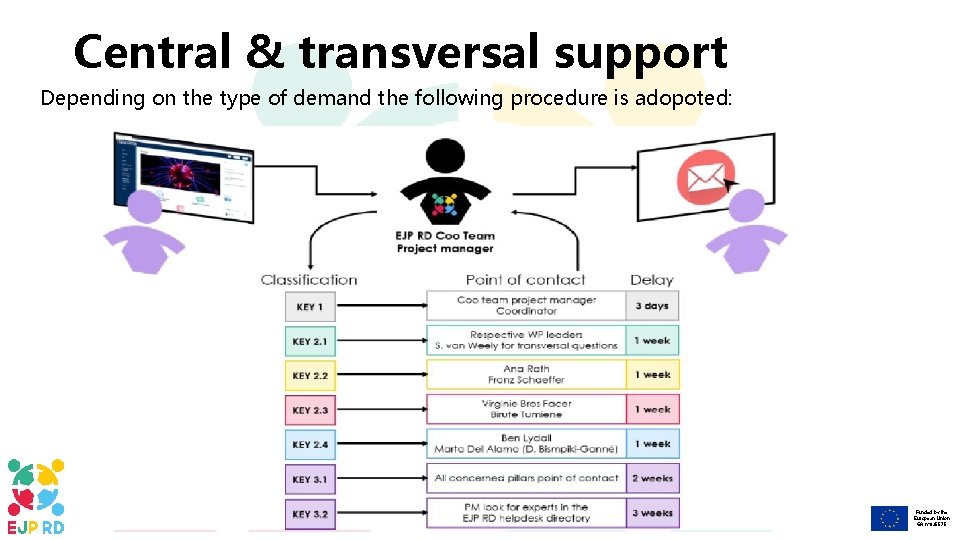
Central & transversal support Depending on the type of demand the following procedure is adopoted: Funded by the European Union GA n° 825575

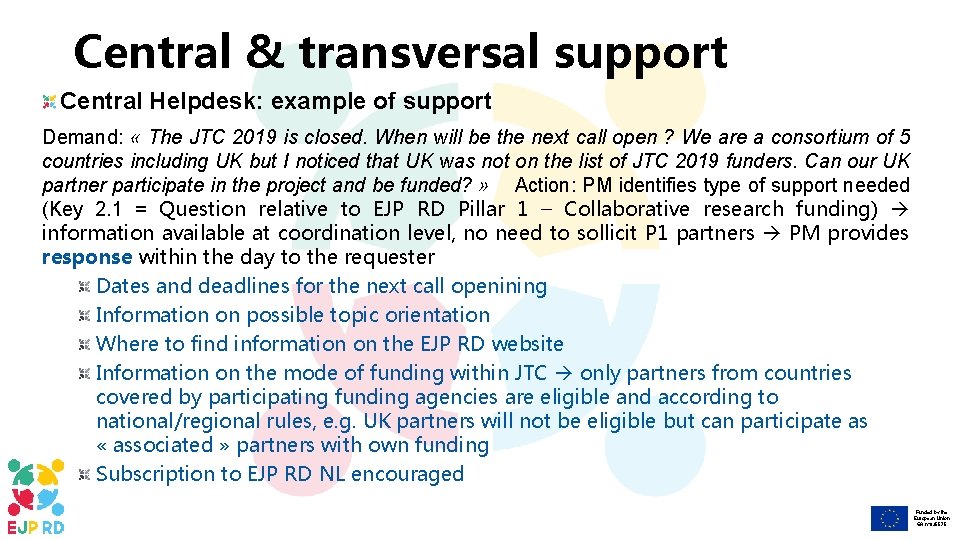
Central & transversal support Central Helpdesk: example of support Demand: « The JTC 2019 is closed. When will be the next call open ? We are a consortium of 5 countries including UK but I noticed that UK was not on the list of JTC 2019 funders. Can our UK partner participate in the project and be funded? » Action: PM identifies type of support needed (Key 2. 1 = Question relative to EJP RD Pillar 1 – Collaborative research funding) information available at coordination level, no need to sollicit P 1 partners PM provides response within the day to the requester Dates and deadlines for the next call openining Information on possible topic orientation Where to find information on the EJP RD website Information on the mode of funding within JTC only partners from countries covered by participating funding agencies are eligible and according to national/regional rules, e. g. UK partners will not be eligible but can participate as « associated » partners with own funding Subscription to EJP RD NL encouraged Funded by the European Union GA n° 825575

Central & transversal support Central Helpdesk: example of support Demand: « I wanted to know if I could receive your advice for designing a clinical trial and obtaining funding. It is a generic drug for an ultra rare disease without treatment. We do not belong to any ERN. What do you recommend? » Action: PM identifies type of support needed (Key 3. 1 = more than one service concerned) most suitable services indentified by PM: ECRIN CT Support Helpdesk + WP 19 (EATRIS) PM contacts responsible persons (experts) within EJP RD experts propose the approach is being communicated to the requester follow up is ensured by the indentified services/infrastructures Doodle a first TC with the PI, ECRIN and EATRIS (and anyone else that may be of help) Request some brief further information (non-confidential) so we can plan the call better: More info on the drug and trial (reminding non-confidential, but we need indication at least) Is there orphan drug designation? If not, what are plans? Is the drug available easily in clinical grade? Do they have clinician involved or do we need to bring one in? They intend to be sponsor for investigator-initiated trial? Funded by the European Union GA n° 825575

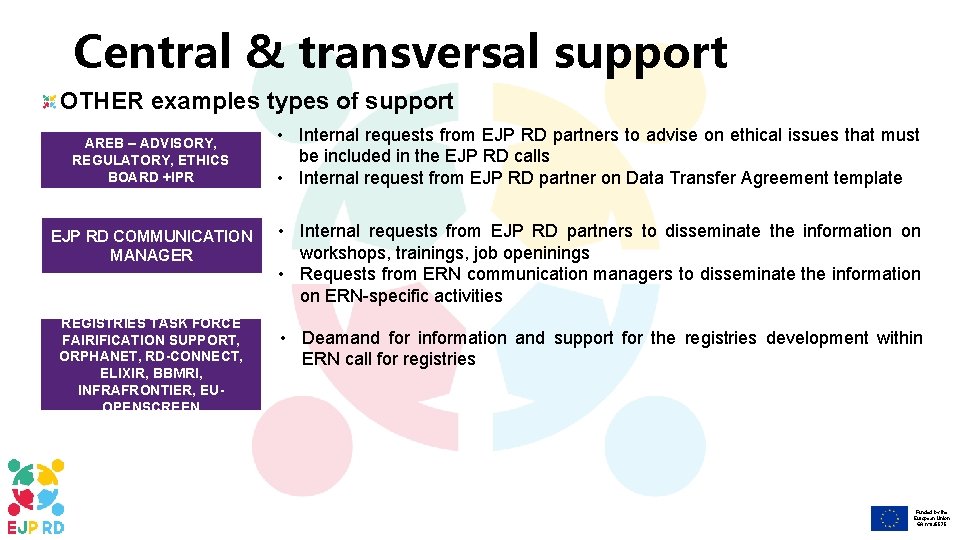
Central & transversal support OTHER examples types of support AREB – ADVISORY, REGULATORY, ETHICS BOARD +IPR EJP RD COMMUNICATION MANAGER REGISTRIES TASK FORCE FAIRIFICATION SUPPORT, ORPHANET, RD-CONNECT, ELIXIR, BBMRI, INFRAFRONTIER, EUOPENSCREEN • Internal requests from EJP RD partners to advise on ethical issues that must be included in the EJP RD calls • Internal request from EJP RD partner on Data Transfer Agreement template • Internal requests from EJP RD partners to disseminate the information on workshops, trainings, job openinings • Requests from ERN communication managers to disseminate the information on ERN-specific activities • Deamand for information and support for the registries development within ERN call for registries Funded by the European Union GA n° 825575

HOW YOU CAN BENEFIT FROM INFRASTRUCTURES/EJP RD SUPPORT IN THE CONTEXT OF THE EJP RD JOINT TRANSNATIONAL CALLS

Joint transnational call 2020 context TOPIC: therapies for rare diseases (pre-clinical) Topic keywords: novel therapies, molecules, screening, repurposing, biomarkers, data analytical methods, pharmacodynamics, human samples, disease models, proof-ofprinciple (pre-clinical) Approach keywords: translability into human, quality & reproducibility, sample quality (and certification), target validity, right tissue, safety profile in models, orphan drug designation, scalability for CTs and manufacturing, risk-based approach, statistical soundness and power, bioinformatics, risk management, FAIR data Evaluation keywords: clarity, credibility, robustness, competence & quality, innovative potential, commercial exploitation, translability, impact on health care, unmet need, involvement of patients, risk management, cost-effectiveness Use of existing EU research infrastructures and IRDi. RC recommended resources is strongly recommented and can be financed within the research project Funded by the European Union GA n° 825575

Joint transnational call 2020 context KEEP IN MIND THAT: You will be evaluated for the translability, quality and soundness of your project No fishing expeditions, if you do not have qualitative samples, data, exprtise in readability and risk assessment e. g. talk to your TTOs or find suitable expertise outside of your institutions(s) Use of existing EU research infrastructures and IRDi. RC recommended resources is strongly recommented and can be financed within the research project: Sometimes (some support) is for free if your country is a partner As subcontractors As partner in your project (not the whole infrastructure) but specific institutions form this infrastructure (check eligibility according to national/regional rules) Funded by the European Union GA n° 825575

Joint transnational call 2020 context Suitable infrastructures: BBMRI Biobanking and Biomolecular Resources Research Infrastructure EATRIS European Infrastructure for Translational Medicine ECRIN European Clinical Research Infrastructure Network ELIXIR The European Life Sciences Infrastructure for Biological Information EU-OPENSCREEN European high-capacity screening network Human Phenotype Ontology INFRAFRONTIER European Infrastructure for Phenotyping, Archiving and Distribution of Mouse Models INSTRUCT Integrated Structural Biology Infrastructure for Europe IRDi. RC recognized resources: Matchmaker Exchange A federated platform to facilitate the matching of cases with similar phenotypic and genotypic profiles RD-Connect An integrated platform connecting databases, registries, biobanks and clinical bioinformatics for rare disease research Orphanet Rare Disease Ontology Horizon 2020 FAIR Data Management Plan Annex 1 in: Recommendations for Improving the Quality of Rare Disease Registries Funded by the European Union GA n° 825575

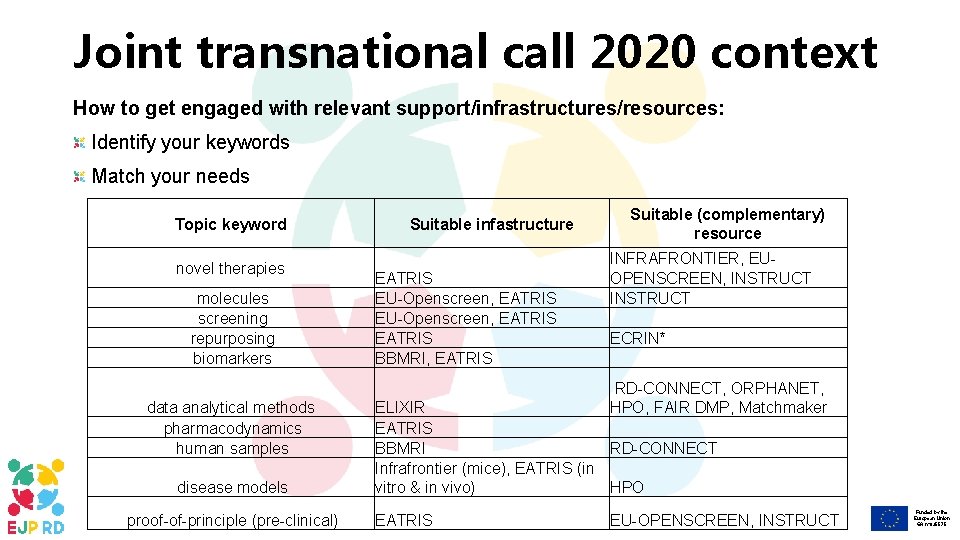
Joint transnational call 2020 context How to get engaged with relevant support/infrastructures/resources: Identify your keywords Match your needs Topic keyword novel therapies molecules screening repurposing biomarkers data analytical methods pharmacodynamics human samples disease models proof-of-principle (pre-clinical) Suitable infastructure EATRIS EU-Openscreen, EATRIS BBMRI, EATRIS Suitable (complementary) resource INFRAFRONTIER, EUOPENSCREEN, INSTRUCT ECRIN* RD-CONNECT, ORPHANET, HPO, FAIR DMP, Matchmaker ELIXIR EATRIS BBMRI RD-CONNECT Infrafrontier (mice), EATRIS (in vitro & in vivo) HPO EATRIS EU-OPENSCREEN, INSTRUCT Funded by the European Union GA n° 825575

Joint transnational call 2020 context How to get engaged with relevant support/infrastructures/resources: Identify your keywords Match your needs Ask for support: Through the EJP RD Central Helpdesk difficulties to identify the best support = general demand Through the EJP RD Central Helpdesk but with tagging pre-identified service already provided within the EJP RD: Innovation support office - EATRIS (WP 19, Pillar 4) Clinical trials support office - ECRIN (WP 20, Pillar 4) FAIRification support (WP 12, Pillar 2) Biobanking – BBMRI (WP 11, Pillar 2) Registry support (WP 11, Pillar 2) Data management support – ELIXIR (WP 11, Pillar 2) Mouse models – INFRAFRONTIER (WP 11, Pillar 2) Molecule screening – EU-OPENSCREEN (WP 11, Pillar 2) Biomarkers – EATRIS (WP 19, Pillar 4) Ontologies, gen-phen relevance – ORPHANET, HPO, RD-CONNECT (WP 11, Pillar 2) System approaches – INSTRUCT, X-OMICS support (WP 11 & WP 13, Pillar 2) Ethics issues – EJP RD AREB Through a direct contact with the infrastructure/service/resource Funded by the European Union GA n° 825575

THANK YOU www. ejprarediseases. org coordination@ejprarediseases. org helpdesk@ejprarediseases. org