Erythrocytes Are Specialized Containers for Transporting Gases erythrocytes

- Slides: 6

Erythrocytes Are Specialized Containers for Transporting Gases erythrocytes are mechanically tough, yet highly-deformable

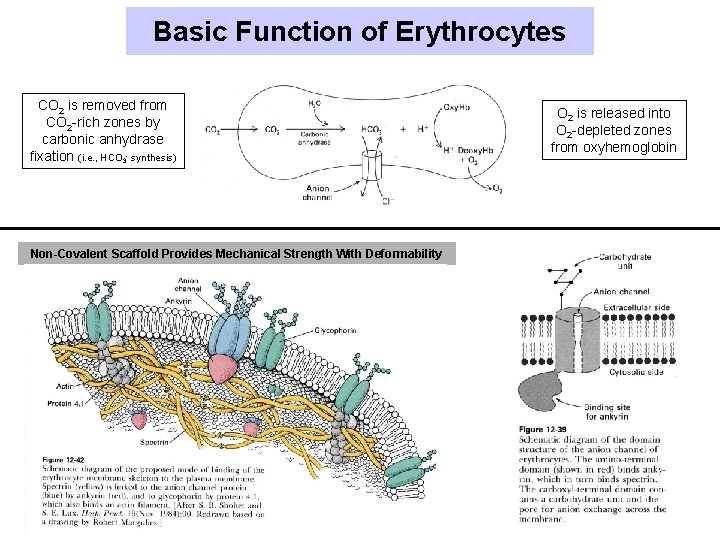
Basic Function of Erythrocytes CO 2 is removed from CO 2 -rich zones by carbonic anhydrase fixation (i. e. , HCO 3 - synthesis) Non-Covalent Scaffold Provides Mechanical Strength With Deformability O 2 is released into O 2 -depleted zones from oxyhemoglobin

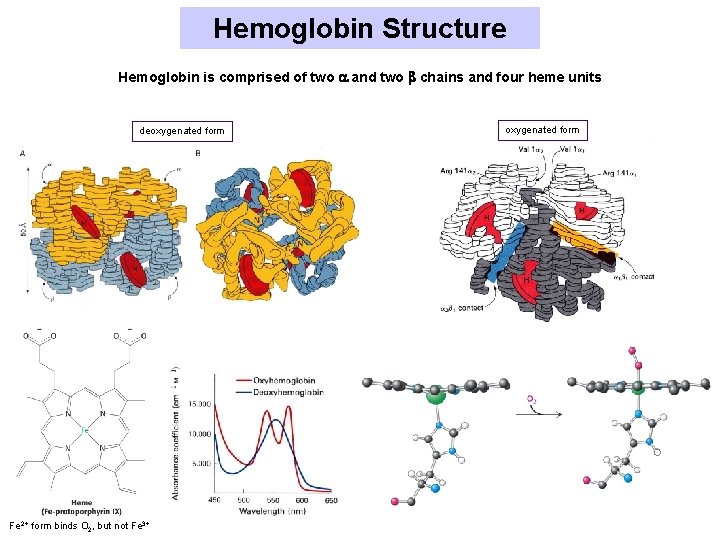
Hemoglobin Structure Hemoglobin is comprised of two and two chains and four heme units deoxygenated form Fe 2+ form binds O 2, but not Fe 3+ oxygenated form

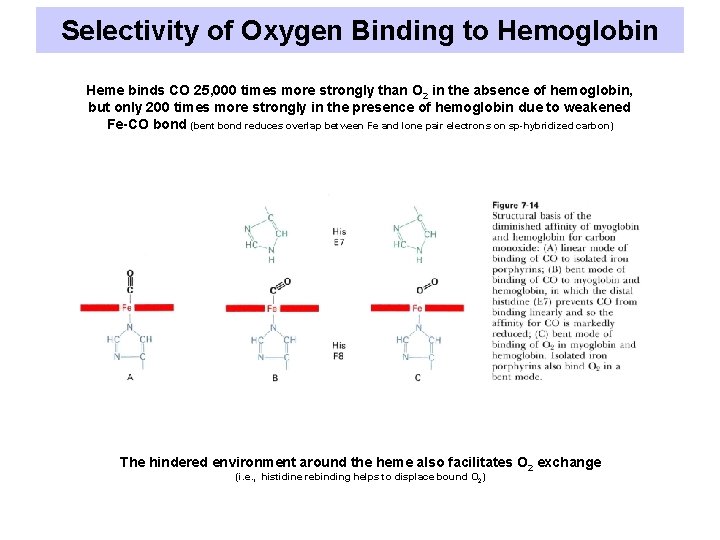
Selectivity of Oxygen Binding to Hemoglobin Heme binds CO 25, 000 times more strongly than O 2 in the absence of hemoglobin, but only 200 times more strongly in the presence of hemoglobin due to weakened Fe-CO bond (bent bond reduces overlap between Fe and lone pair electrons on sp-hybridized carbon) The hindered environment around the heme also facilitates O 2 exchange (i. e. , histidine rebinding helps to displace bound O 2)

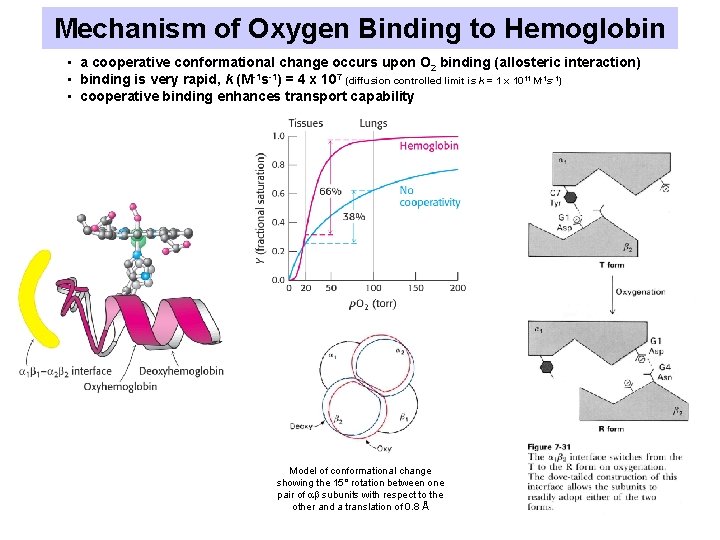
Mechanism of Oxygen Binding to Hemoglobin • a cooperative conformational change occurs upon O 2 binding (allosteric interaction) • binding is very rapid, k (M-1 s-1) = 4 x 107 (diffusion controlled limit is k = 1 x 1011 M-1 s-1) • cooperative binding enhances transport capability Model of conformational change showing the 15° rotation between one pair of subunits with respect to the other and a translation of 0. 8 Å

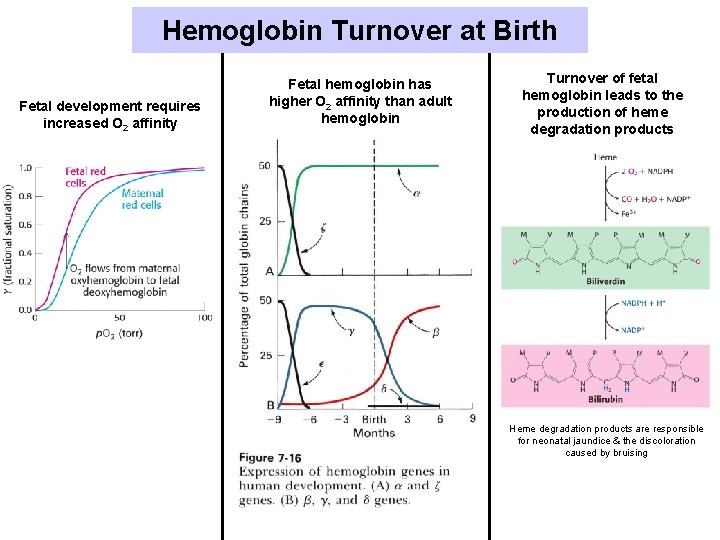
Hemoglobin Turnover at Birth Fetal development requires increased O 2 affinity Fetal hemoglobin has higher O 2 affinity than adult hemoglobin Turnover of fetal hemoglobin leads to the production of heme degradation products Heme degradation products are responsible for neonatal jaundice & the discoloration caused by bruising