Energy D 2 Explain how energy is transferred









- Slides: 23

Energy

D 2 Explain how energy is transferred by conduction, convection and radiation.

D 3/D 5 D 3 - Describe energy transformations among heat, light, electricity and motion. D 5 - Explain how electricity is used to produce heat and light in incandescent bulbs and heating elements.

Warming up … Define “energy. ” Energy is defined as the ability to do work. Work is an activity involving a force and movement in the direction of the force

There are five categories of energy. � The five main forms of energy are: 1. 2. 3. 4. 5. Mechanical Nuclear Chemical Electromagnetic Heat

Subclasses of Energy Mechanical ◦ This is energy of motion that is used to perform work

Nuclear Energy The nucleus of an atom is the source of nuclear energy.

Nuclear Energy Fission: When the nucleus splits, nuclear energy is released in the form of heat energy and light energy. Fusion: When nuclei collide at high speeds and join, releasing energy.

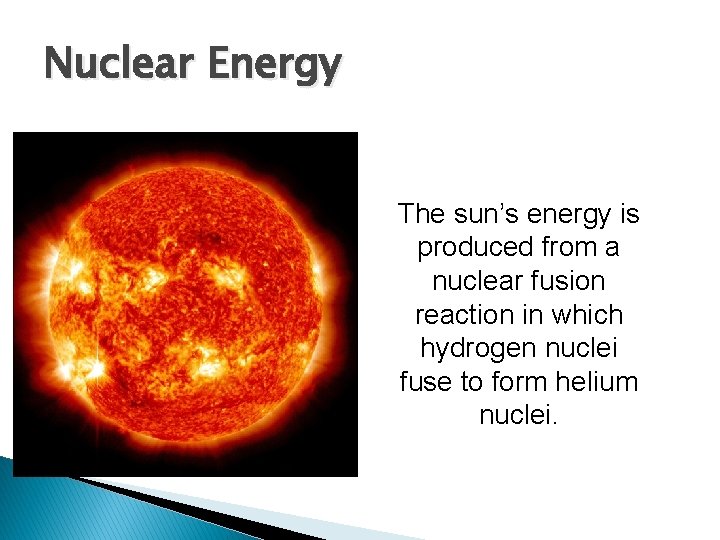
Nuclear Energy The sun’s energy is produced from a nuclear fusion reaction in which hydrogen nuclei fuse to form helium nuclei.

Nuclear Energy The nuclear energy produces energy through nuclear fission.

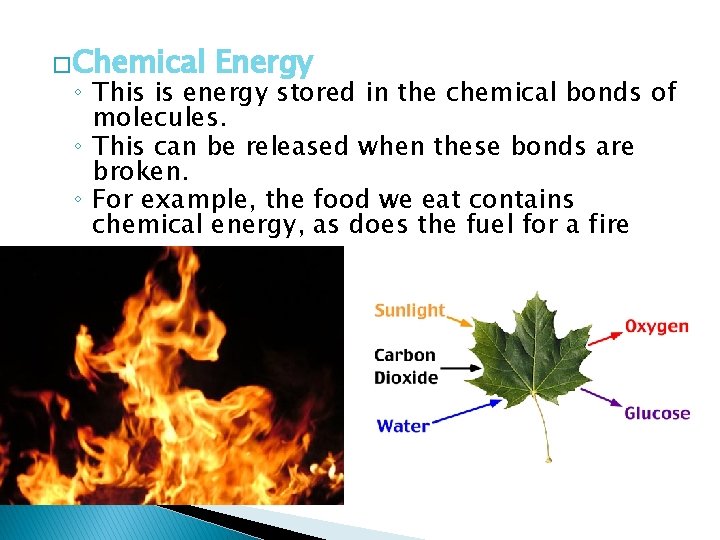
�Chemical Energy ◦ This is energy stored in the chemical bonds of molecules. ◦ This can be released when these bonds are broken. ◦ For example, the food we eat contains chemical energy, as does the fuel for a fire

Chemical energy can be transferred to the surroundings as heat, light, motion and sound when that energy is released

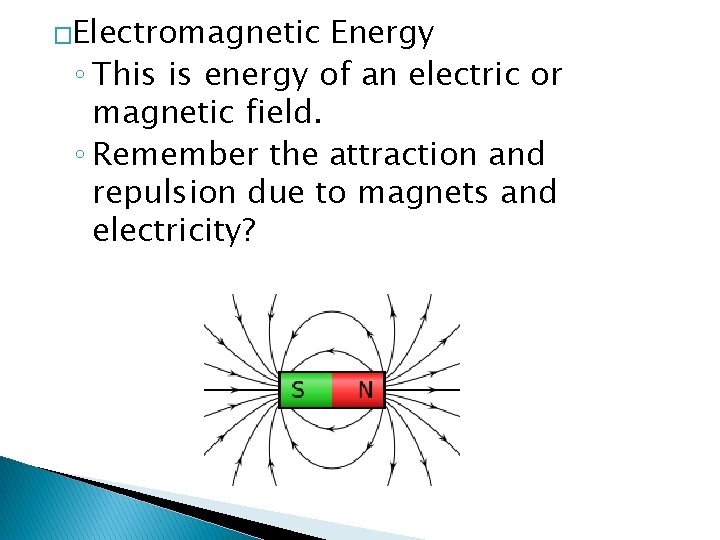
�Electromagnetic Energy ◦ This is energy of an electric or magnetic field. ◦ Remember the attraction and repulsion due to magnets and electricity?

Electromagnetic Energy � Power lines carry electromagnetic energy into your home in the form of electricity. � What is the definition of “electricity? ”

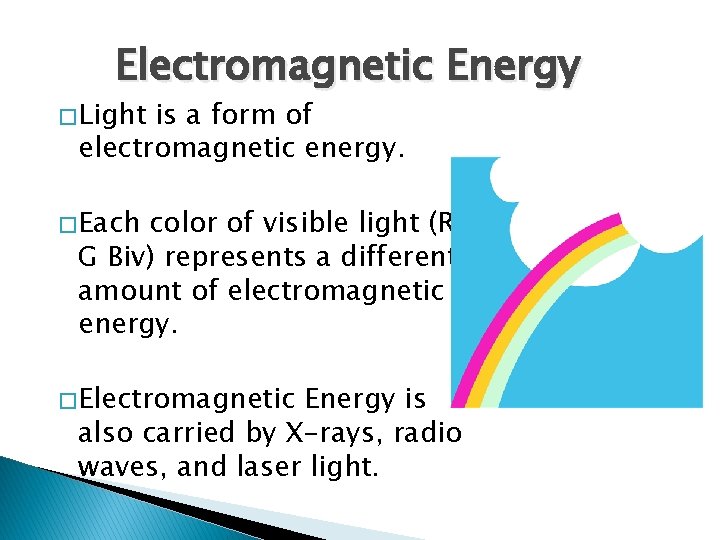
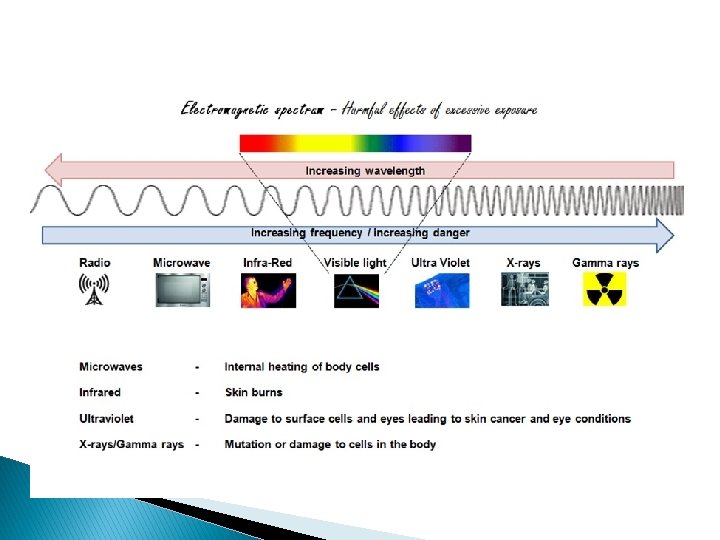
Electromagnetic Energy � Light is a form of electromagnetic energy. � Each color of visible light (Roy G Biv) represents a different amount of electromagnetic energy. � Electromagnetic Energy is also carried by X-rays, radio waves, and laser light.


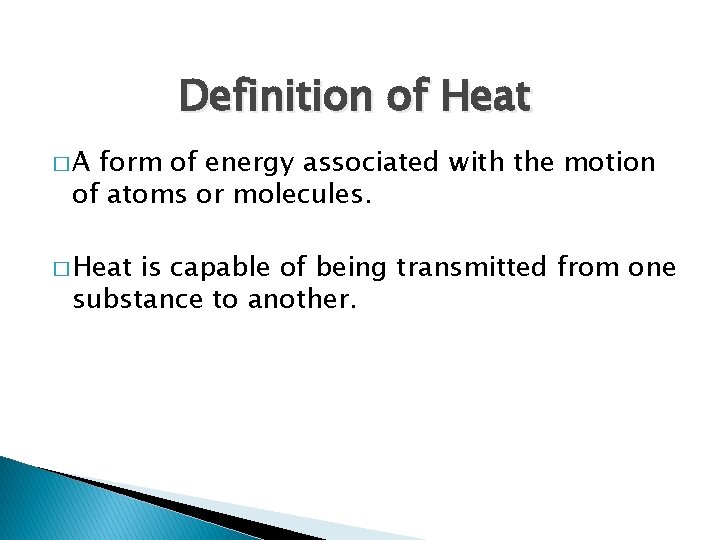
Definition of Heat �A form of energy associated with the motion of atoms or molecules. � Heat is capable of being transmitted from one substance to another.

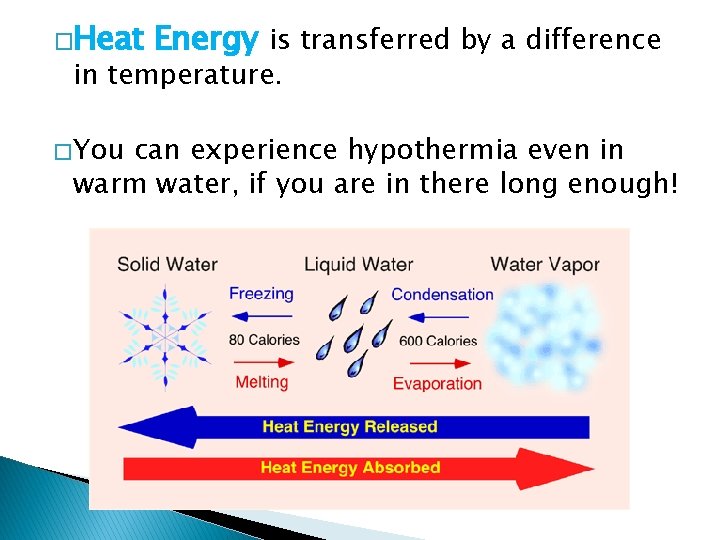
�Heat Energy is transferred by a difference in temperature. � You can experience hypothermia even in warm water, if you are in there long enough!

Temperature and Heat Temperature and heat are NOT THE SAME.

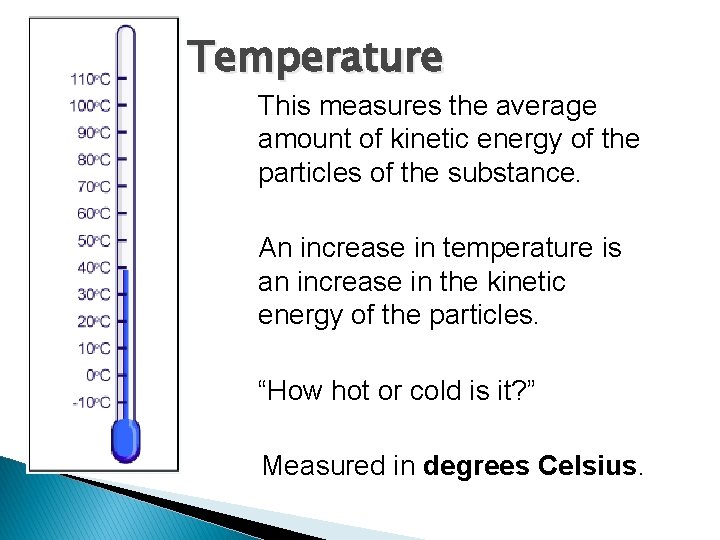
Temperature This measures the average amount of kinetic energy of the particles of the substance. An increase in temperature is an increase in the kinetic energy of the particles. “How hot or cold is it? ” Measured in degrees Celsius.

Heat The amount of energy, measured in joules (J). Heat, when added to a substance, can either change its temperature, or change its phase.

A swimming pool at 30°C is at a lower temperature than a cup of tea at 80°C. BUT the swimming pool contains more water, so it stores more thermal energy or heat.

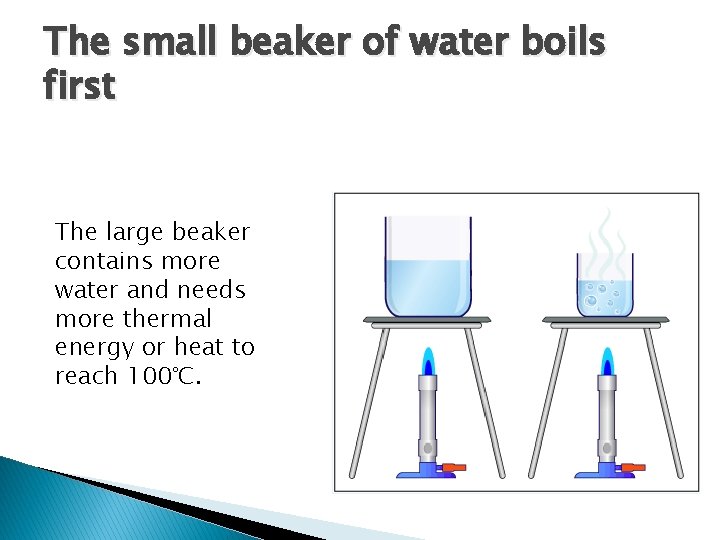
The small beaker of water boils first The large beaker contains more water and needs more thermal energy or heat to reach 100°C.