Heat is in physics energy transferred from one




- Slides: 14


Heat, is in physics, energy transferred from one part of a substance to another, or from one body to another, by virtue of a difference in temperature. Heat is energy in transit; it flows from a substance at a higher temperature that is placed in contact with a substance at a lower temperature, raising the temperature of the latter and lowering that of the former, provided the volume of the bodies remains constant. Heat does not flow from a lower to a higher temperature unless work done.

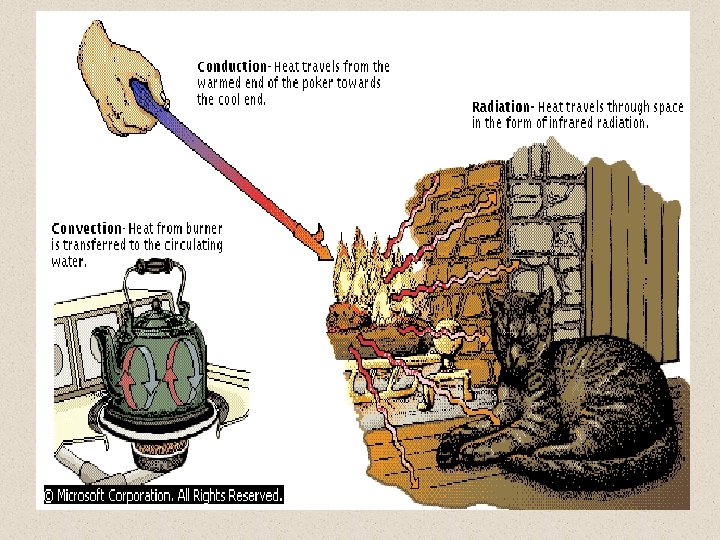
TRANSFER OF HEAT Heat can be transferred by three processes: conduction, convection, and radiation. Conduction is the transfer of heat through a solid object; it is this process that makes the handle of a poker hot, even if only the tip is in the fire. Convection transfers heat through the exchange of hot and cold molecules; this is the process through which water in a kettle becomes uniformly hot even though only the bottom of the kettle contacts the flame. Radiation is the transfer of heat via electromagnetic (usually infrared) radiation; this is the principal mechanism through which a fire warms a room.

TRANSFER OF HEAT The physical processes by which heat transfer occurs are conduction and radiation. A third process, which also involves the motion of matter, is called convection. Conduction requires physical contact between the bodies or portions of bodies exchanging heat, but radiation does not require contact or the presence of any matter between the bodies. Convection occurs through the motion of a liquid or gas in contact with matter at a different temperature.


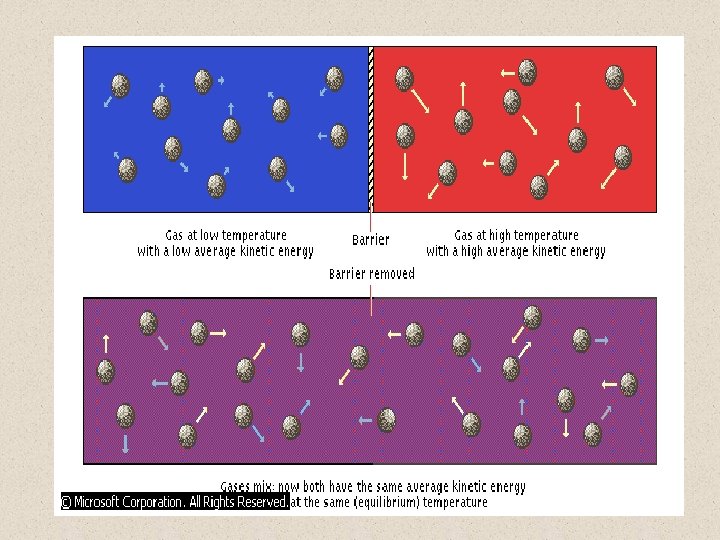
THERMAL EQUILIBRIUM Two identical gases at different temperatures are separated by a barrier. The hotter gas consists of molecules with a larger average kinetic energy than the molecules of the cooler gas. When the gases are combined, the temperature of the mixture settles at an equilibrium temperature that is between the temperature of the hot gas and the temperature of the cold gas. The heat flows from the warmer gas to the colder gas until the average kinetic energies of the two gases are



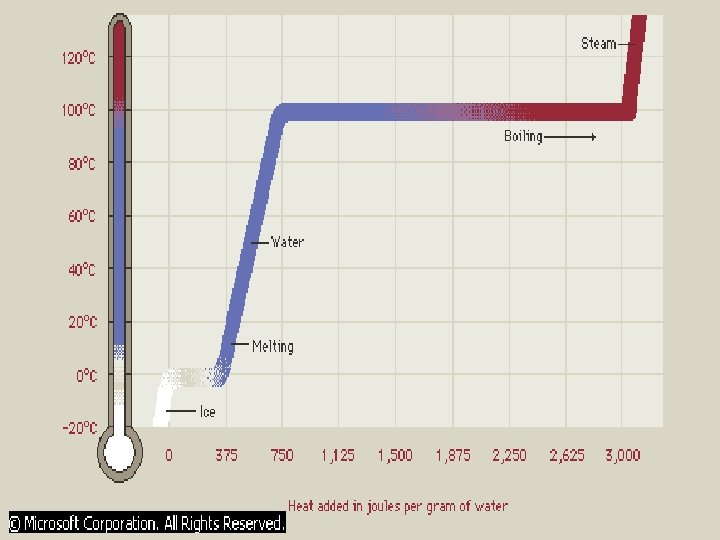
The graph represents the temperature change that occurs when heat is added to water. At 0° C and at 100° C, you can add heat to water without changing its temperature. This “latent heat” breaks bonds that hold the molecules together but does not increase their kinetic energy. Note that approximately seven times more heat must be added to evaporate one gram of water than to melt it. This is represented by the relative lengths of the horizontal portions of the graph. The slopes of the inclined lines represent the number of degrees that the temperature changes for each joule of heat that is added to one gram. The “specific heat capacity” of water is 4, 200 joules per kilogram per kelvin. In other words it takes 4, 200 joules of energy to raise the temperature of one kilogram of water by one degree Celsius or one kelvin.

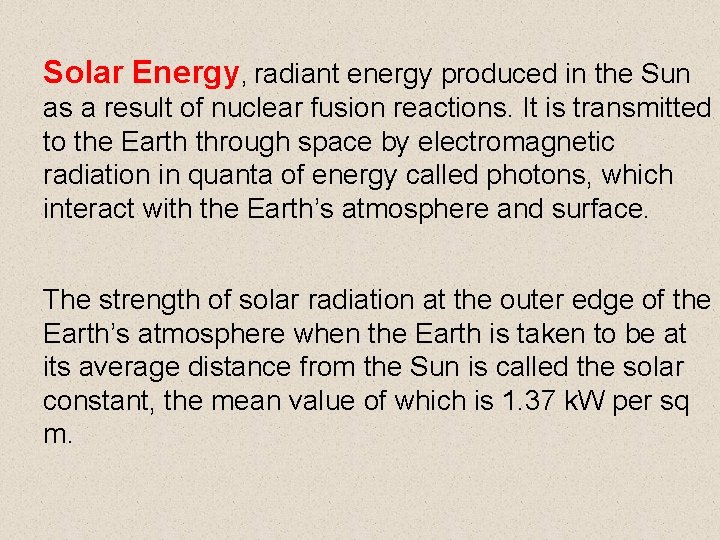
Solar Energy, radiant energy produced in the Sun as a result of nuclear fusion reactions. It is transmitted to the Earth through space by electromagnetic radiation in quanta of energy called photons, which interact with the Earth’s atmosphere and surface. The strength of solar radiation at the outer edge of the Earth’s atmosphere when the Earth is taken to be at its average distance from the Sun is called the solar constant, the mean value of which is 1. 37 k. W per sq m.

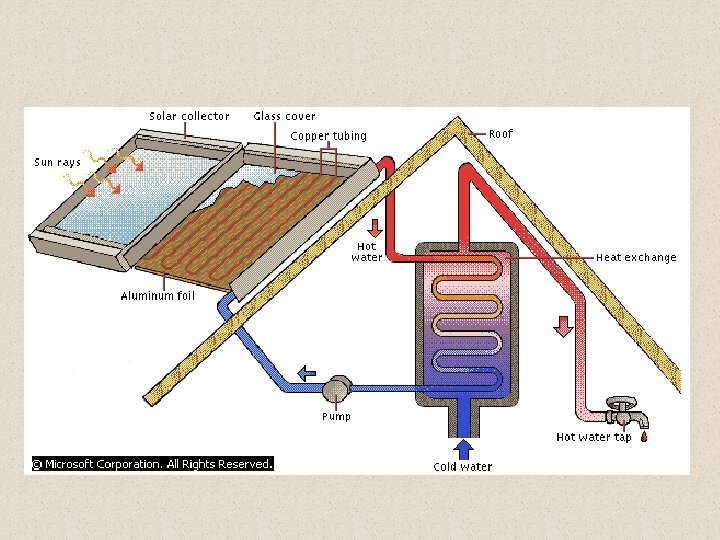
SOLAR HEATING DEVICES Flat plate collectors utilize the sun’s energy to warm a carrier fluid, which in turn provides usable heat to a household. The carrier fluid, which in this case is water, flows through copper tubing in the solar collector, and in the process absorbs some of the sun’s energy. Next, the carrier fluid moves to the heat exchange, where the carrier fluid warms water that is used by the household. Finally, a pump moves the carrier fluid back to the solar collector to repeat the cycle.


SOLAR HOME In this solar home in Corrales, New Mexico, United States, a flat plate solar collector (lower right) provides energy to heat water pumped by the windmill. The water is stored in large drums at the side of the home.

" Microsoft® Encarta® Encyclopedia 2000. © 1993 -1999 Microsoft Corporation. All rights reserved.