Energy Changes in Nuclear Reactions Energy and mass

- Slides: 7

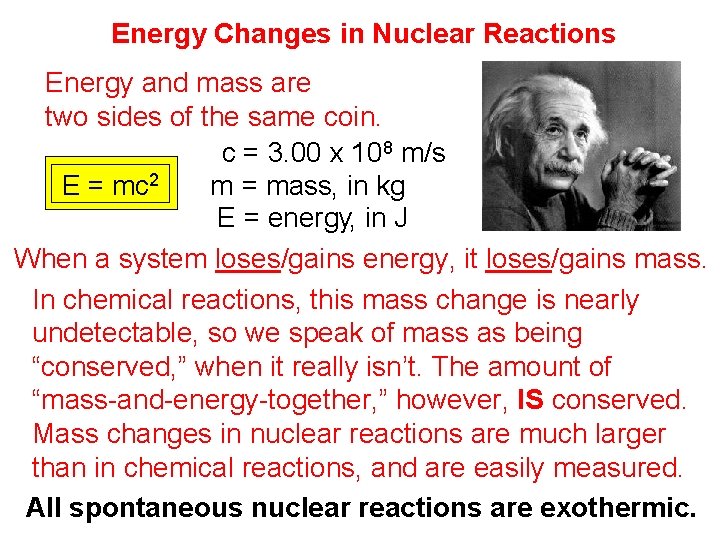
Energy Changes in Nuclear Reactions Energy and mass are two sides of the same coin. c = 3. 00 x 108 m/s E = mc 2 m = mass, in kg E = energy, in J When a system loses/gains energy, it loses/gains mass. In chemical reactions, this mass change is nearly undetectable, so we speak of mass as being “conserved, ” when it really isn’t. The amount of “mass-and-energy-together, ” however, IS conserved. Mass changes in nuclear reactions are much larger than in chemical reactions, and are easily measured. All spontaneous nuclear reactions are exothermic.

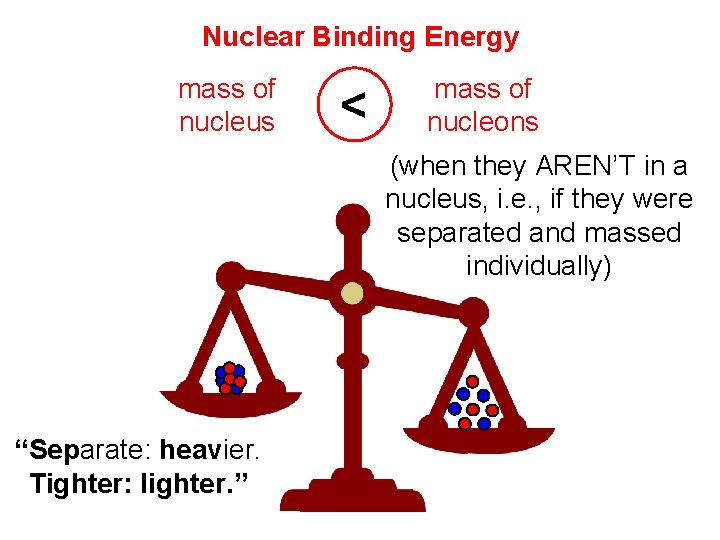
Nuclear Binding Energy mass of nucleus < mass of nucleons (when they AREN’T in a nucleus, i. e. , if they were separated and massed individually) “Separate: heavier. Tighter: lighter. ”

mass of mass defect = constituent – nucleus nucleons (or “mass deficiency”) This “missing” mass is converted into energy, which is used to hold the nucleus together. rest masses: n 0 = 1. 00866 amu = 1. 67493 x 10– 24 g p+ = 1. 00728 amu = 1. 67262 x 10– 24 g e– = 0. 0005486 amu = 9. 113 x 10– 28 g

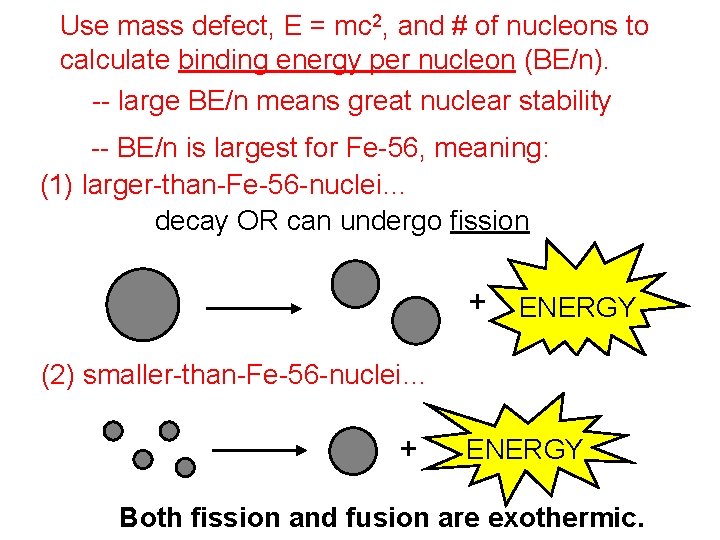
Use mass defect, E = mc 2, and # of nucleons to calculate binding energy per nucleon (BE/n). -- large BE/n means great nuclear stability -- BE/n is largest for Fe-56, meaning: (1) larger-than-Fe-56 -nuclei… decay OR can undergo fission + ENERGY (2) smaller-than-Fe-56 -nuclei… can undergo fusion + ENERGY Both fission and fusion are exothermic.

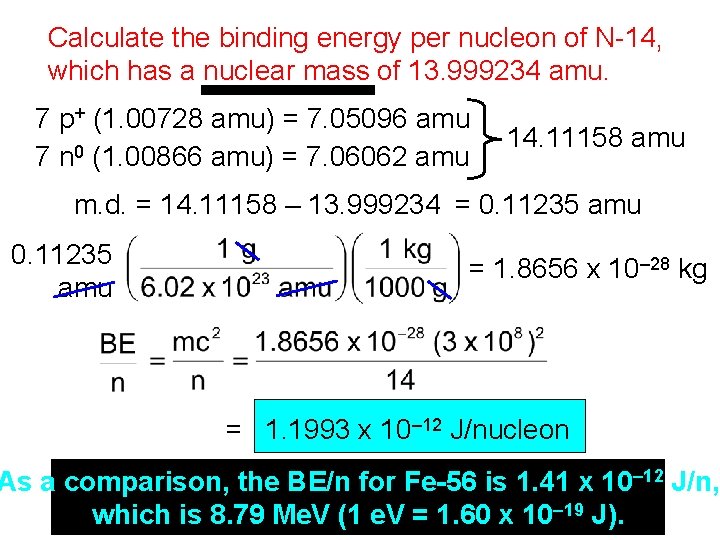
Calculate the binding energy per nucleon of N-14, which has a nuclear mass of 13. 999234 amu. 7 p+ (1. 00728 amu) = 7. 05096 amu 7 n 0 (1. 00866 amu) = 7. 06062 amu 14. 11158 amu m. d. = 14. 11158 – 13. 999234 = 0. 11235 amu = 1. 8656 x 10– 28 kg = 1. 1993 x 10– 12 J/nucleon As a comparison, the BE/n for Fe-56 is 1. 41 x 10– 12 J/n, which is 8. 79 Me. V (1 e. V = 1. 60 x 10– 19 J).

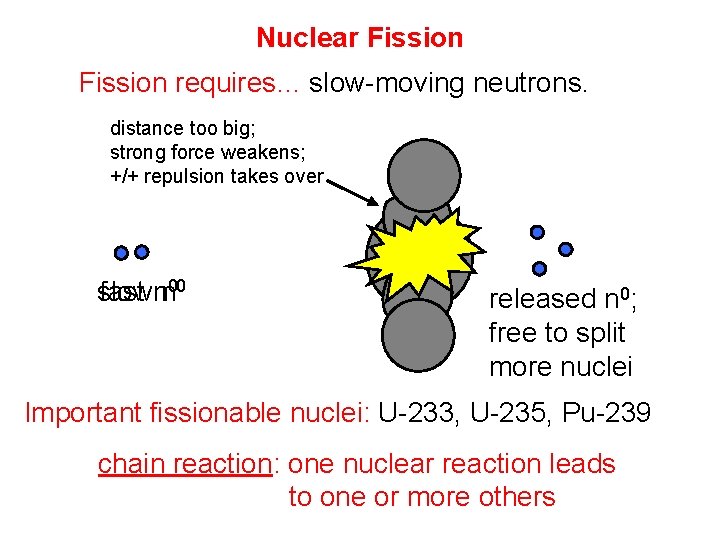
Nuclear Fission requires… slow-moving neutrons. distance too big; strong force weakens; +/+ repulsion takes over slow fast nn 00 released n 0; free to split more nuclei Important fissionable nuclei: U-233, U-235, Pu-239 chain reaction: one nuclear reaction leads to one or more others

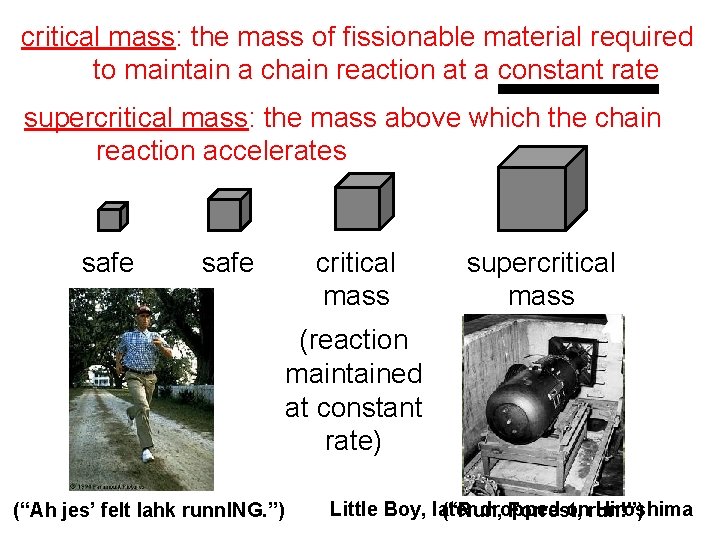
critical mass: the mass of fissionable material required to maintain a chain reaction at a constant rate supercritical mass: the mass above which the chain reaction accelerates safe critical mass supercritical mass (reaction maintained at constant rate) (“Ah jes’ felt lahk runn. ING. ”) Little Boy, later dropped onrun!”) Hiroshima (“Run, Forrest,