Elephant Toothpaste Aimee Gonzalez Materials Graduated cylinder or
















- Slides: 17

Elephant Toothpaste Aimee Gonzalez

Materials ❏ ❏ ❏ ❏ ❏ Graduated cylinder or 16 ounce soda bottle Hydrogen peroxide Dry yeast Potassium iodide Liquid dishwashing soap Food coloring Small cups Safety goggles Gloves

Safety Precautions ❏ This Experiment should be done outside. ❏ Wear proper protective equipment including gloves, lab coat, and safety glasses when preparing and performing this demonstration. Concentrated hydrogen peroxide and potassium iodide can cause skin and eye irritation. ❏ Disposal: Remaining solution can be flushed down drain with plenty of water.

Step 1 Pour hydrogen peroxide solution into the graduated cylinder.

Step 2 Squirt in a few drops of dishwashing soap

Step 3 Place 5 -10 drops of food coloring into the cylinder to make the foam colorful.

Step 4 Add potassium iodide solution into the graduated cylinder, watch to see what happens

Trial # 1 Trial # 2 Trial # 3 6% of hydrogen peroxide 12% of hydrogen peroxide 3% of hydrogen peroxide Dish Soap Dry Yeast Potassium iodide Dish Soap Food coloring Potassium iodide

Trial 1 ❏ Pour 100 ml of 6% of Hydrogen Peroxide into the graduated cylinder ❏ Add a squeeze of dishwashing soap ❏ Add 1 teaspoon of potassium iodide ❏ Wait to see what happens

Trial 2 ❏ Pour 150 ml of 12% of Hydrogen Peroxide into the graduated cylinder ❏ Squeeze a few drops of dishwashing soap ❏ Add a few drops of food dye ❏ Add 2 teaspoons of potassium iodide ❏ Wait to see what happens

Trail 3 ❏ Pour 200 ml of 3% Hydrogen Peroxide into the graduated cylinder ❏ Squeeze a small amount of dishwashing soap ❏ Add a few drops of food dye ❏ Add 3 teaspoons of potassium iodide ❏ One packet of dry yeast, mixed with hot water ❏ Wait to see what happens

What I learned ❏ Percentage of hydrogen peroxide ❏ Potassium iodide - powerful calyast

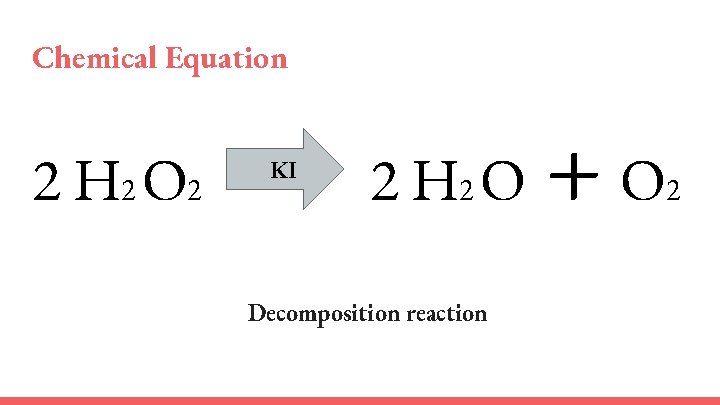
Chemical Equation 2 H 2 O 2 KI 2 H 2 O Decomposition reaction +O 2

Explanation ● The iodide ion (KI) is used as a catalyst to decompose H 2 O 2, liberating water, oxygen and heat. ● The yeast acted as a catalyst to remove the oxygen from the hydrogen peroxide. ● Exothermic Reaction – created both foam, and heat.

Real World ❏ Hydrogen peroxide is used to heal wounds

Stoichiometry

THANK YOU
 Elephant toothpaste reactants and products
Elephant toothpaste reactants and products Volumetric glassware and routine glassware
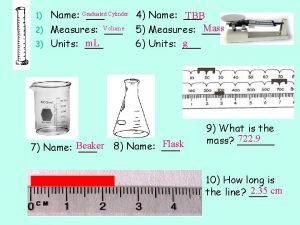
Volumetric glassware and routine glassware What measurement does a graduated cylinder use
What measurement does a graduated cylinder use Measuring graduated cylinder practice
Measuring graduated cylinder practice Back titration antacid
Back titration antacid Graduated cylinder
Graduated cylinder Beaker function chemistry
Beaker function chemistry Graduated cylinder us customary units
Graduated cylinder us customary units Lastt
Lastt Graduated cylinder vs volumetric flask
Graduated cylinder vs volumetric flask Target graduated cylinder
Target graduated cylinder How much leaves and twigs an adult elephant can eat
How much leaves and twigs an adult elephant can eat What is a key stone species
What is a key stone species Themes of shooting an elephant
Themes of shooting an elephant Crushed mouse brains as toothpaste
Crushed mouse brains as toothpaste Chemical reaction of toothpaste
Chemical reaction of toothpaste A name symbol or other feature
A name symbol or other feature Toothpaste image
Toothpaste image