Electrolytic Cell Involves non spontaneous redox reactions Use



- Slides: 8

Electrolytic Cell • Involves non spontaneous redox reactions – Use external power supply to drive the redox reaction (like a battery or plug into wall) – Amount of E needed to drive the reaction depends on the half reactions that make up the cell • Eocell will be negative • Ex. include electroplating and electrolysis of molten salts to the M (s) 2 Na. Cl (molten) → 2 Na (s) + Cl 2 (g)

Electrolytic Cell, Parts • Same as galvanic, still have – Electrolytes – Electrodes (cathode and anode) – Salt bridge • Still have Aut. O and Ca. R – Anode gets oxidized – Cathode gets reduced

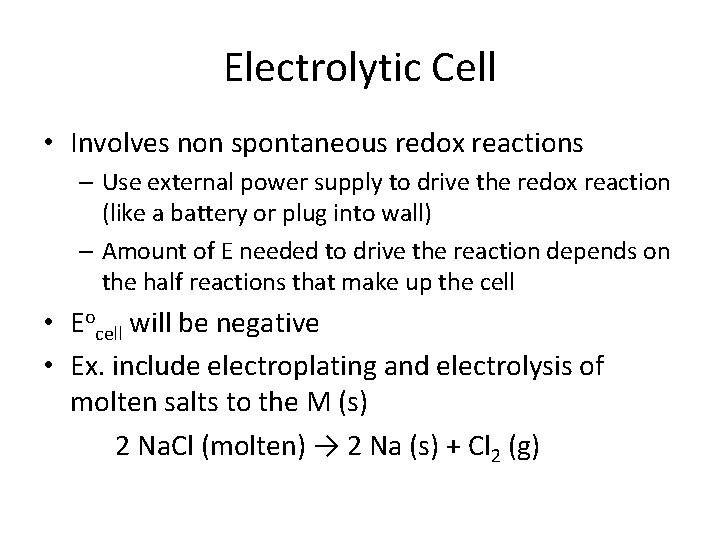
How does it work? Have to put energy into an electrolytic cell to drive the redox rxn (non spont) – Need to connect it to an external power supply (like a battery/galvanic cell) The - end of battery (anode) hooks to cathode in EC The + end of battery (cathode) hooks to anode in EC

Electrolysis of Water • Zap H 2 O to decompose it to elements 2 H 2 O → 2 H 2 + O 2 Who is oxidized? Who is reduced? Write balanced half reactions. (note: H 2 half reaction is basic, O 2 is acidic)

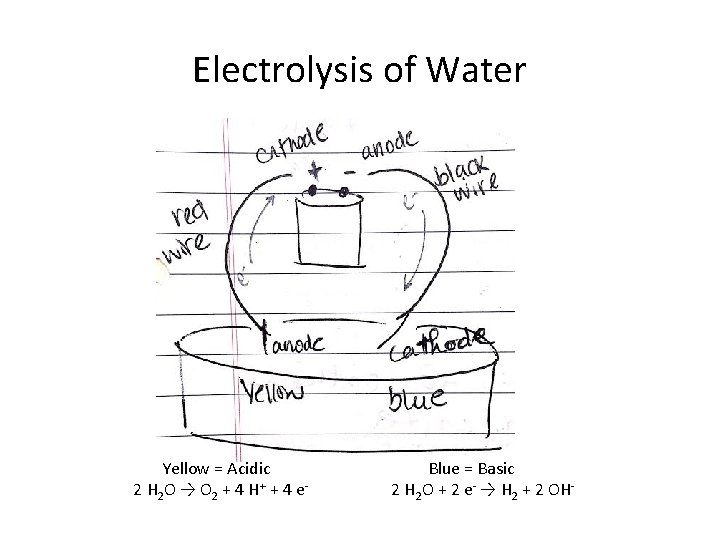
Electrolysis of Water Yellow = Acidic 2 H 2 O → O 2 + 4 H+ + 4 e- Blue = Basic 2 H 2 O + 2 e- → H 2 + 2 OH-

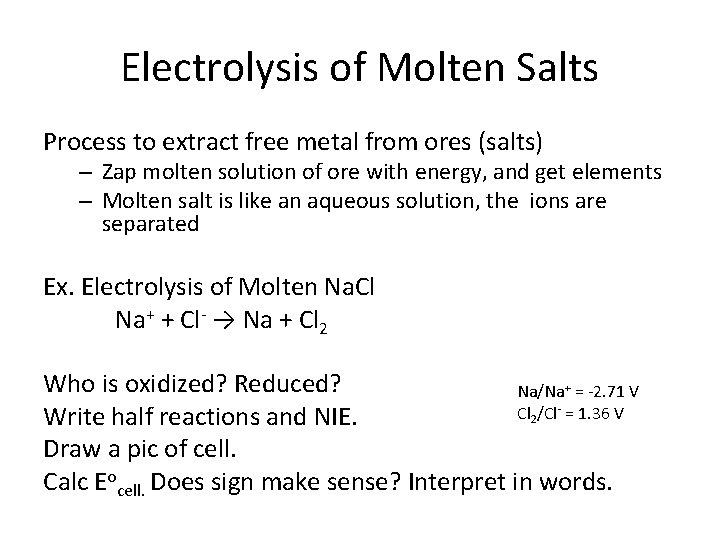
Electrolysis of Molten Salts Process to extract free metal from ores (salts) – Zap molten solution of ore with energy, and get elements – Molten salt is like an aqueous solution, the ions are separated Ex. Electrolysis of Molten Na. Cl Na+ + Cl- → Na + Cl 2 Who is oxidized? Reduced? Na/Na+ = -2. 71 V Cl 2/Cl- = 1. 36 V Write half reactions and NIE. Draw a pic of cell. Calc Eocell. Does sign make sense? Interpret in words.

Metal in Ore Gets Reduced Nonmetal Gets Oxidized • If you have a mixture of salts like KCl and Na. Cl, which metal will get reduced first? Na+ + e- → Na Eo = -2. 71 V K+ + e- → K Eo = -2. 92 V • Which anion would get oxidized more easily?

Quantitative Aspect of Electrolysis • See sheet