Electrochemistry Cell Potential and the Nernst Equation The

- Slides: 11

Electrochemistry Cell Potential and the Nernst Equation

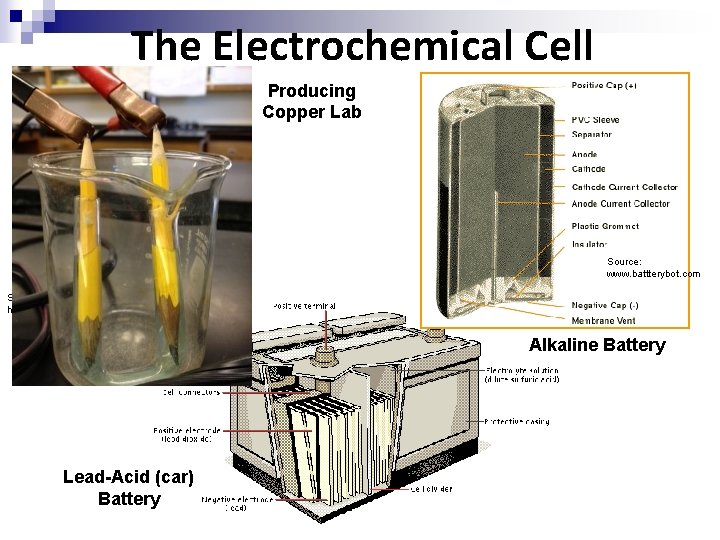
The Electrochemical Cell Producing Copper Lab Source: www. battterybot. com Source: http: //www. reuk. co. uk Alkaline Battery Lead-Acid (car) Battery

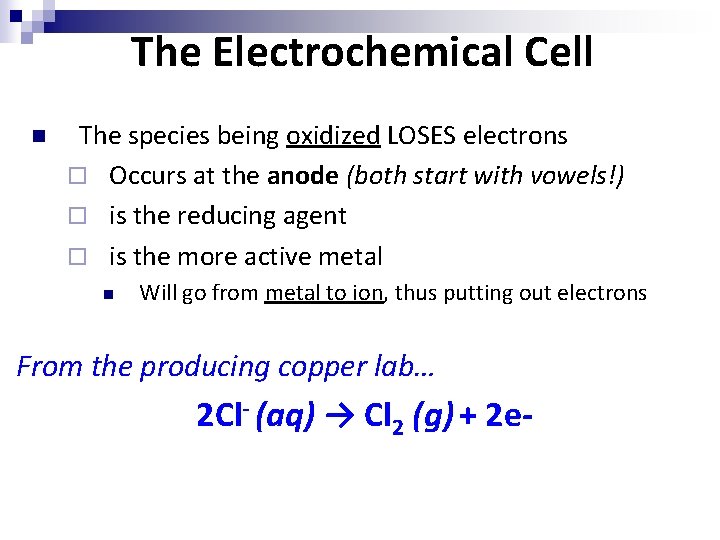
The Electrochemical Cell n The species being oxidized LOSES electrons ¨ Occurs at the anode (both start with vowels!) ¨ is the reducing agent ¨ is the more active metal n Will go from metal to ion, thus putting out electrons From the producing copper lab… 2 Cl- (aq) → Cl 2 (g) + 2 e-

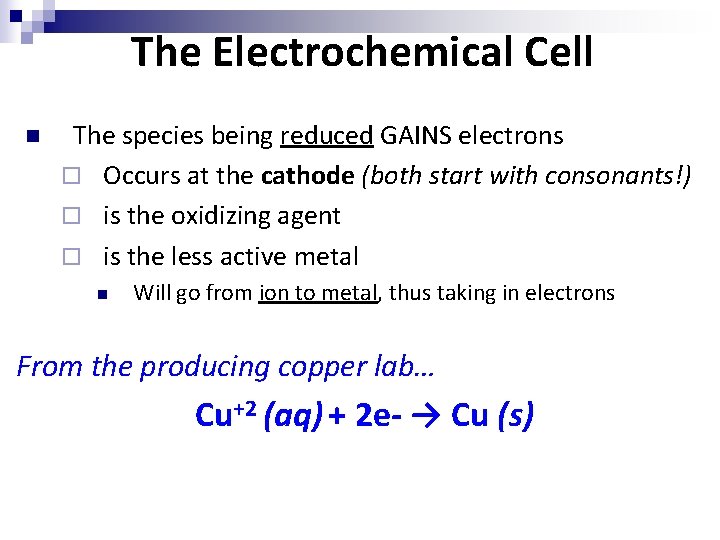
The Electrochemical Cell n The species being reduced GAINS electrons ¨ Occurs at the cathode (both start with consonants!) ¨ is the oxidizing agent ¨ is the less active metal n Will go from ion to metal, thus taking in electrons From the producing copper lab… Cu+2 (aq) + 2 e- → Cu (s)

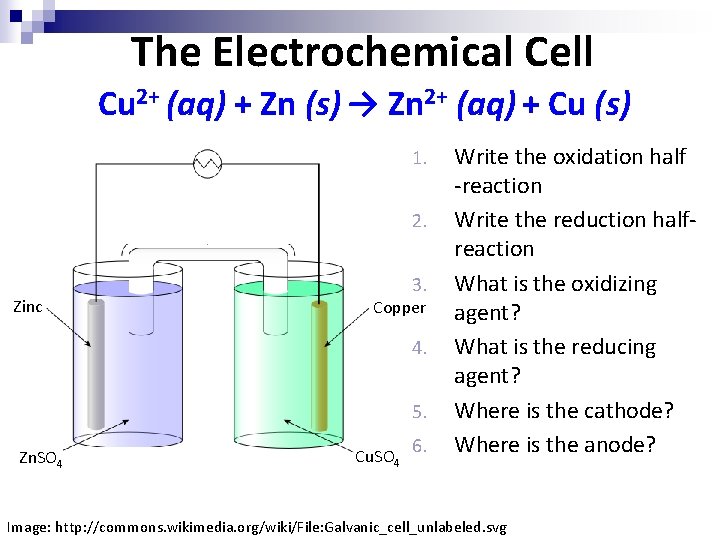
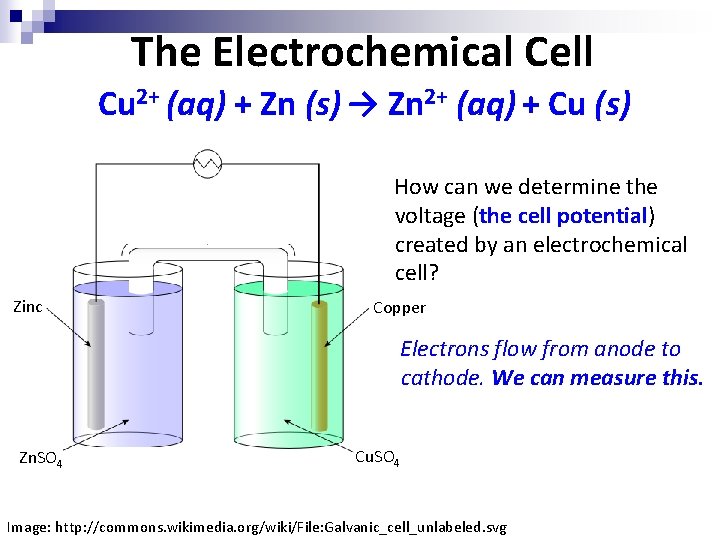
The Electrochemical Cell Cu 2+ (aq) + Zn (s) → Zn 2+ (aq) + Cu (s) 1. 2. Zinc 3. Copper 4. 5. Zn. SO 4 Cu. SO 4 6. Write the oxidation half -reaction Write the reduction halfreaction What is the oxidizing agent? What is the reducing agent? Where is the cathode? Where is the anode? Image: http: //commons. wikimedia. org/wiki/File: Galvanic_cell_unlabeled. svg

The Electrochemical Cell Cu 2+ (aq) + Zn (s) → Zn 2+ (aq) + Cu (s) How can we determine the voltage (the cell potential) created by an electrochemical cell? Zinc Copper Electrons flow from anode to cathode. We can measure this. Zn. SO 4 Cu. SO 4 Image: http: //commons. wikimedia. org/wiki/File: Galvanic_cell_unlabeled. svg

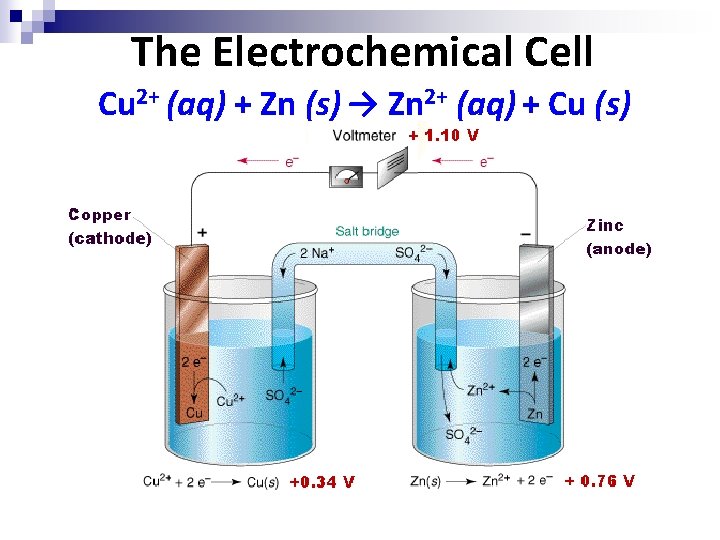
The Electrochemical Cell Cu 2+ (aq) + Zn (s) → Zn 2+ (aq) + Cu (s)

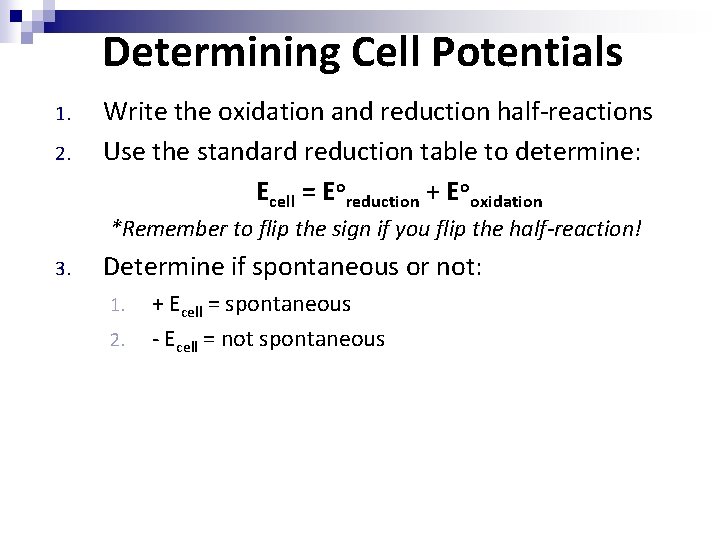
Determining Cell Potentials 1. 2. Write the oxidation and reduction half-reactions Use the standard reduction table to determine: Ecell = Eoreduction + Eooxidation *Remember to flip the sign if you flip the half-reaction! 3. Determine if spontaneous or not: 1. 2. + Ecell = spontaneous - Ecell = not spontaneous

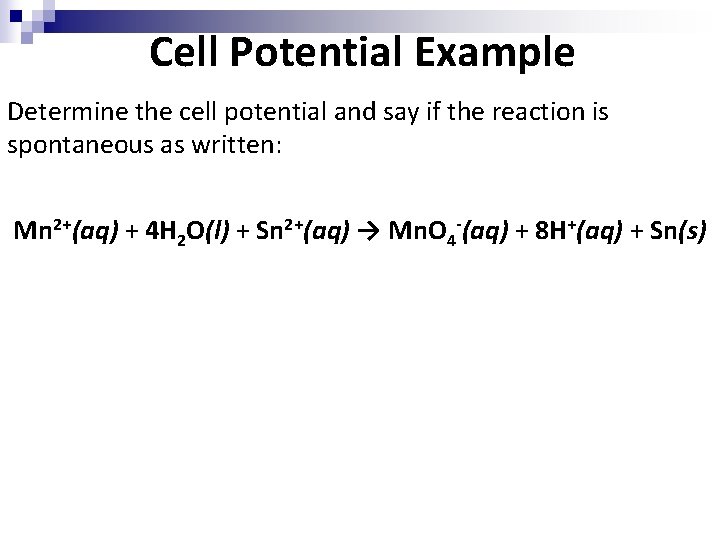
Cell Potential Example Determine the cell potential and say if the reaction is spontaneous as written: Mn 2+(aq) + 4 H 2 O(l) + Sn 2+(aq) → Mn. O 4 -(aq) + 8 H+(aq) + Sn(s)

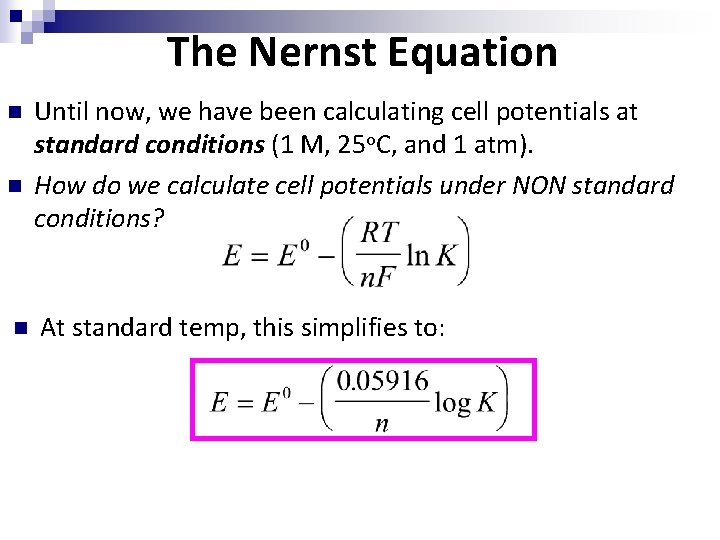
The Nernst Equation n Until now, we have been calculating cell potentials at standard conditions (1 M, 25 o. C, and 1 atm). How do we calculate cell potentials under NON standard conditions? At standard temp, this simplifies to:

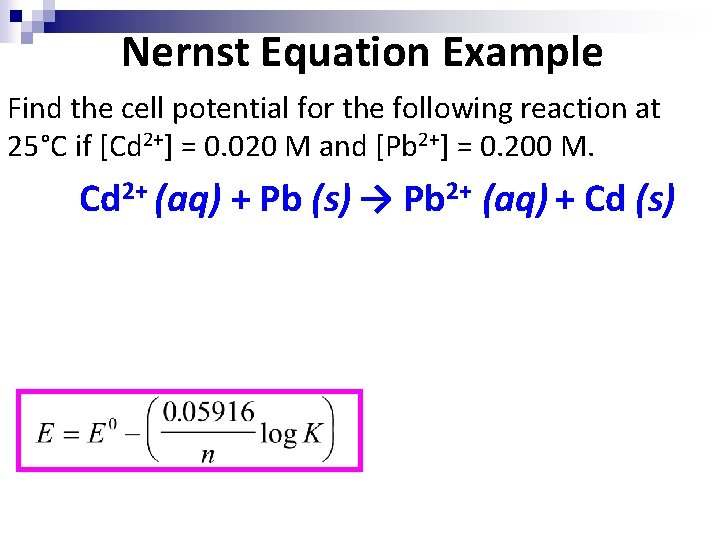
Nernst Equation Example Find the cell potential for the following reaction at 25°C if [Cd 2+] = 0. 020 M and [Pb 2+] = 0. 200 M. Cd 2+ (aq) + Pb (s) → Pb 2+ (aq) + Cd (s)