Electric Charge Unit 11 Electrostatics Properties of Electric





- Slides: 11

Electric Charge Unit 11 Electrostatics

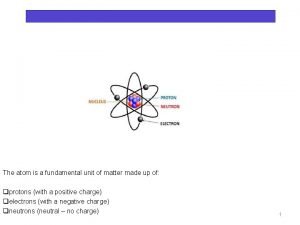
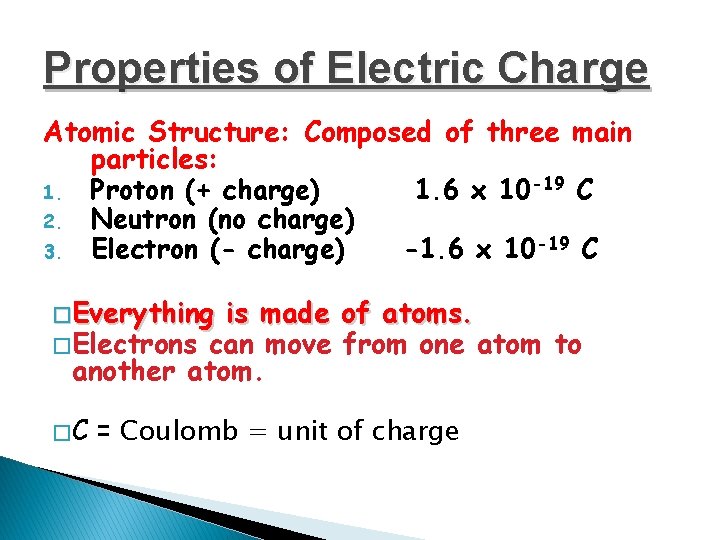
Properties of Electric Charge Atomic Structure: Composed of three main particles: 1. Proton (+ charge) 1. 6 x 10 -19 C 2. Neutron (no charge) 3. Electron (- charge) -1. 6 x 10 -19 C � Everything is made of atoms. � Electrons can move from one atom to another atom. �C = Coulomb = unit of charge

How do charges behave? � Charges behave like magnets. � 2 positive charges or 2 negative charges put together will repel each other. � Put a positive charge near a negative charge and they will attract each other. � A charged object may even attract a neutral one. � Opposites attract……Like repel

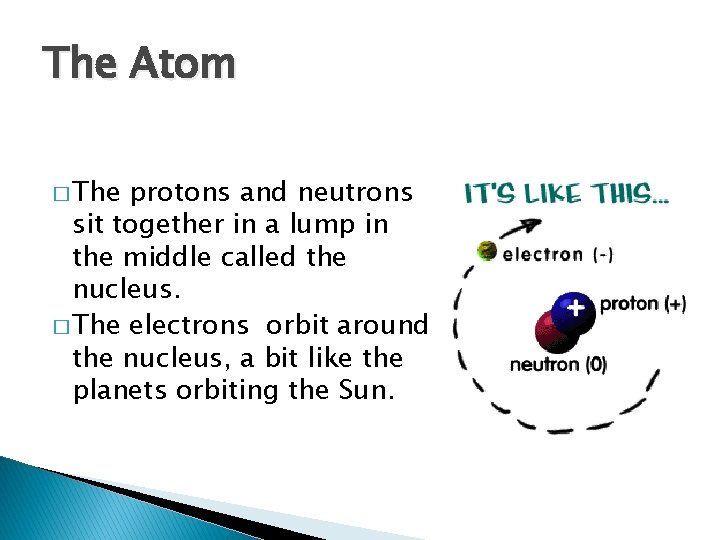
The Atom � The protons and neutrons sit together in a lump in the middle called the nucleus. � The electrons orbit around the nucleus, a bit like the planets orbiting the Sun.

Check for understanding � Positive charged particle? � Negatively charged particle? � Particle with no charge? � Like charges ______. � Unlike charges _____.

Static Electricity � The temporary building up of charge on an object. � Some atoms hold e- more tightly than others. � Ex. Your shoes and Carpet � Most things have the same number of electrons and protons in them. � They don’t have any overall charge.

Static Electricity � Static electricity is caused when certain materials are rubbed against each other. � Electrons can be rubbed off one material and on to another. � The material that has extra electrons is now negatively charged � The material which has lost electrons is positively charged.

Check for understanding � What is static electricity? � When would you have more static electricity in your laundry: when you dry a load of all of the same textures of clothes or when you dry a load of mixed clothes? � How does a dryer sheet work to decrease static electricity?

Make a 1 Minute Video Based on demos presented on https: //www. youtube. com/watch? v=Vi. ZNg. U-Yt-Y

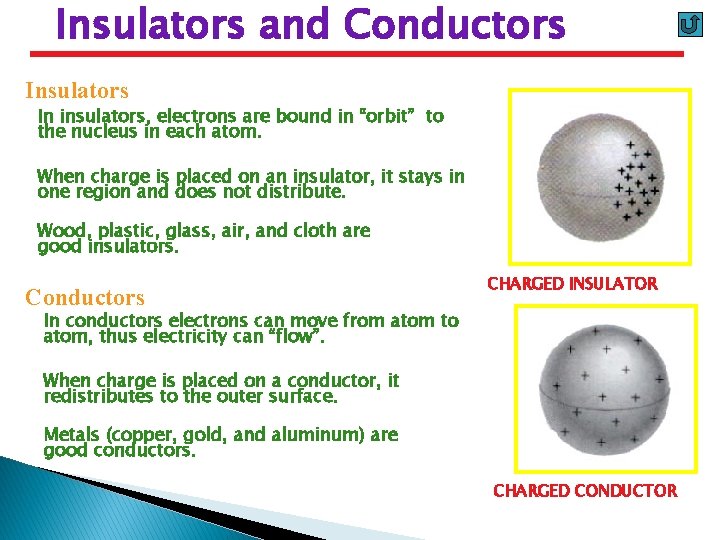
Insulators and Conductors Insulators In insulators, electrons are bound in “orbit” to the nucleus in each atom. When charge is placed on an insulator, it stays in one region and does not distribute. Wood, plastic, glass, air, and cloth are good insulators. Conductors CHARGED INSULATOR In conductors electrons can move from atom to atom, thus electricity can “flow”. When charge is placed on a conductor, it redistributes to the outer surface. Metals (copper, gold, and aluminum) are good conductors. CHARGED CONDUCTOR

Check for understanding � How are insulators and conductors different? � If electricity can be conducted by a substance than can also heat be conducted? Why/not � Is there a difference in the number of electrons in a conductor vs an insulator? � What is generally the relationship of protons to electrons for conductors? � Are conductors attracted to charged objects? � List 2 examples of insulators and 2 examples of conductors.
 Difference between charge and electric charge
Difference between charge and electric charge Electrons flowing
Electrons flowing Chapter 21 electric charge and electric field
Chapter 21 electric charge and electric field Chapter 21 electric charge and electric field
Chapter 21 electric charge and electric field Units of charge
Units of charge Units of a charge
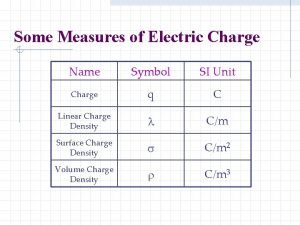
Units of a charge Volume charge density symbol name
Volume charge density symbol name Units of electric charge
Units of electric charge Units of charge
Units of charge Coulomb's law force between two point charges
Coulomb's law force between two point charges Charge q unit
Charge q unit Electric energy
Electric energy