Efficacy Paul Meyers MD ViceChairman Dept of Pediatrics




- Slides: 21

Efficacy Paul Meyers, MD Vice-Chairman, Dept of Pediatrics Memorial Sloan-Kettering Cancer Center 2023. 01 1

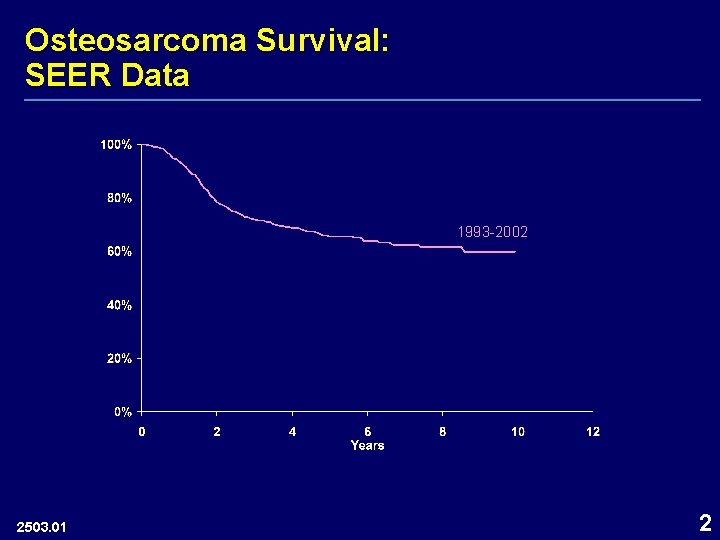
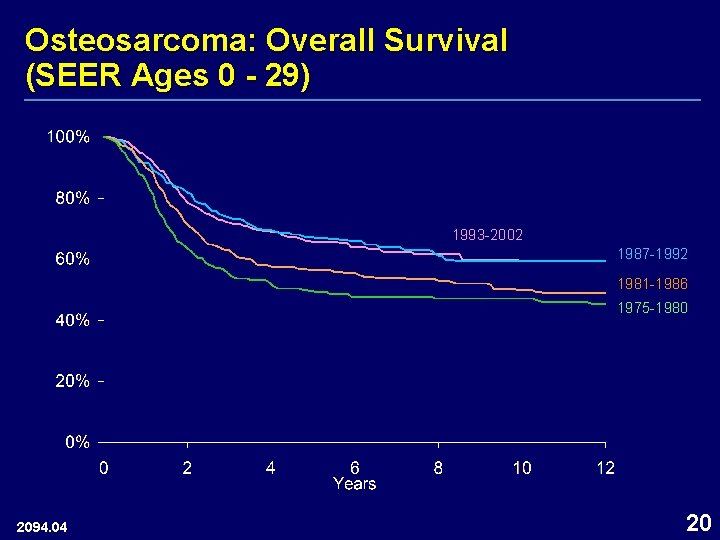
Osteosarcoma Survival: SEER Data 1993 -2002 2503. 01 2

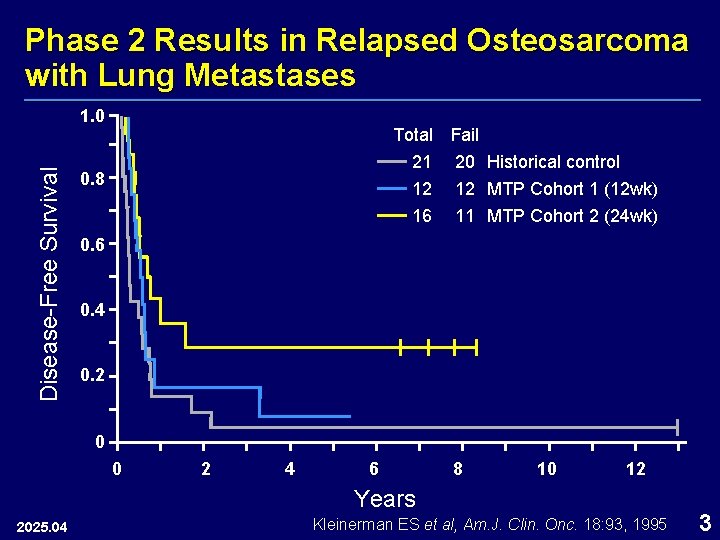
Phase 2 Results in Relapsed Osteosarcoma with Lung Metastases Disease-Free Survival 1. 0 Total Fail 21 20 Historical control 0. 8 12 16 12 MTP Cohort 1 (12 wk) 11 MTP Cohort 2 (24 wk) 0. 6 0. 4 0. 2 0 0 2 4 6 8 10 12 Years 2025. 04 Kleinerman ES et al, Am. J. Clin. Onc. 18: 93, 1995 3

Landmark Phase 3 Trial NCI-sponsored, Cooperative Group study – Designed and conducted independently of corporate sponsor Largest study completed in osteosarcoma – 178 sites – 1/3 of eligible incident cases in US Newly diagnosed (within 30 days; age <31) – 678 non-metastatic resectable disease – 115 with more advanced disease 2027. 01 4

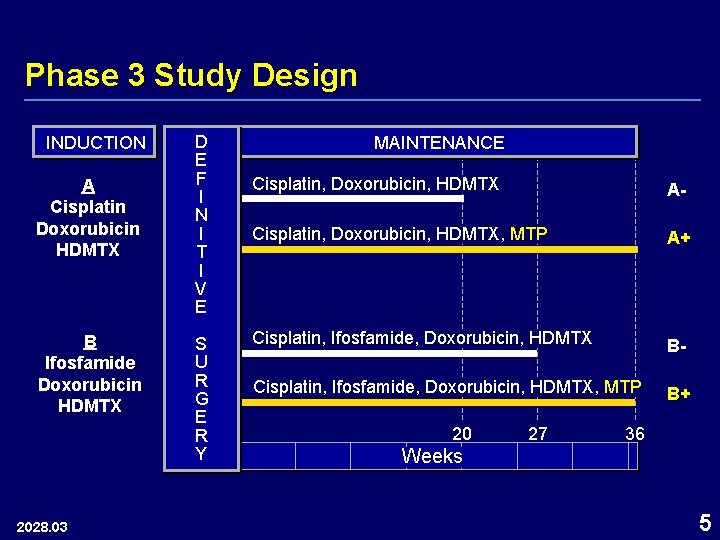
Phase 3 Study Design INDUCTION A Cisplatin Doxorubicin HDMTX B Ifosfamide Doxorubicin HDMTX 2028. 03 D E F I N I T I V E S U R G E R Y MAINTENANCE Cisplatin, Doxorubicin, HDMTX A- Cisplatin, Doxorubicin, HDMTX, MTP A+ Cisplatin, Ifosfamide, Doxorubicin, HDMTX B- Cisplatin, Ifosfamide, Doxorubicin, HDMTX, MTP B+ 20 27 36 Weeks 5

Phase 3 Study Endpoints DFS – Putative surrogate for OS – Prospectively defined primary endpoint – “To determine whether MTP can improve DFS…” – “To compare the results of a prospective, randomized trial of two chemotherapeutic regimens…” (A vs. B) Survival – “To improve the survival of patients with osteogenic sarcoma” 2034. 02 6

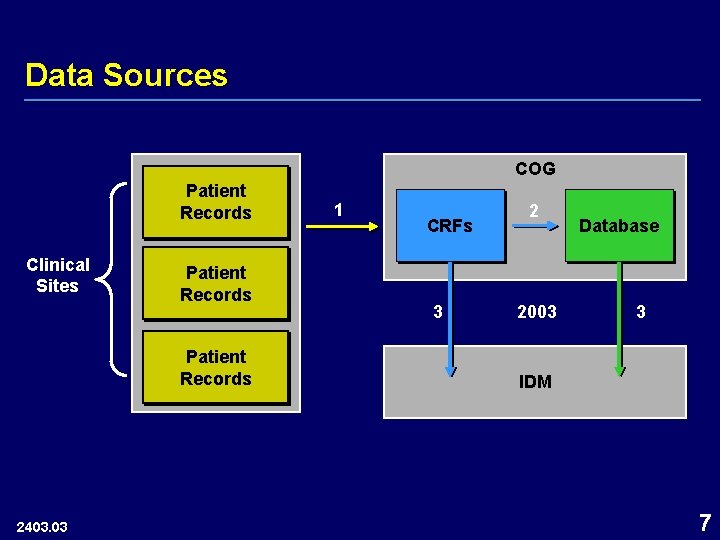
Data Sources COG Patient Records Clinical Sites Patient Records 2403. 03 1 CRFs 3 2 2003 Database 3 IDM 7

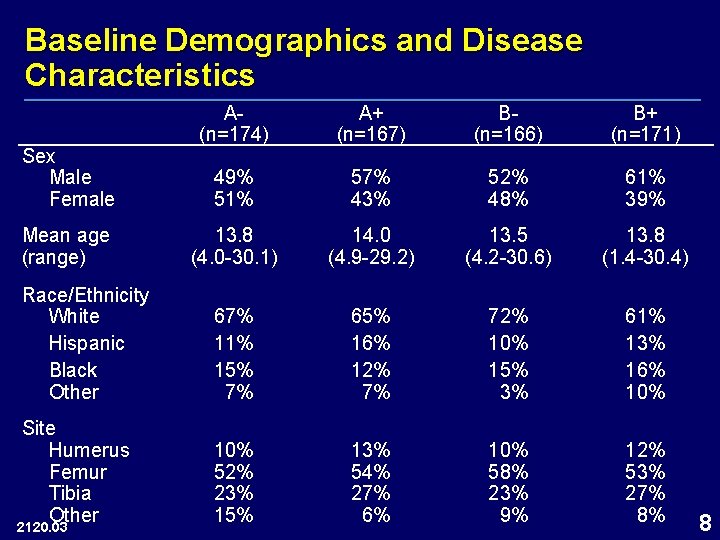
Baseline Demographics and Disease Characteristics A(n=174) A+ (n=167) B(n=166) B+ (n=171) 49% 51% 57% 43% 52% 48% 61% 39% 13. 8 (4. 0 -30. 1) 14. 0 (4. 9 -29. 2) 13. 5 (4. 2 -30. 6) 13. 8 (1. 4 -30. 4) Race/Ethnicity White Hispanic Black Other 67% 11% 15% 7% 65% 16% 12% 7% 72% 10% 15% 3% 61% 13% 16% 10% Site Humerus Femur Tibia Other 10% 52% 23% 15% 13% 54% 27% 6% 10% 58% 23% 9% 12% 53% 27% 8% Sex Male Female Mean age (range) 2120. 03 8

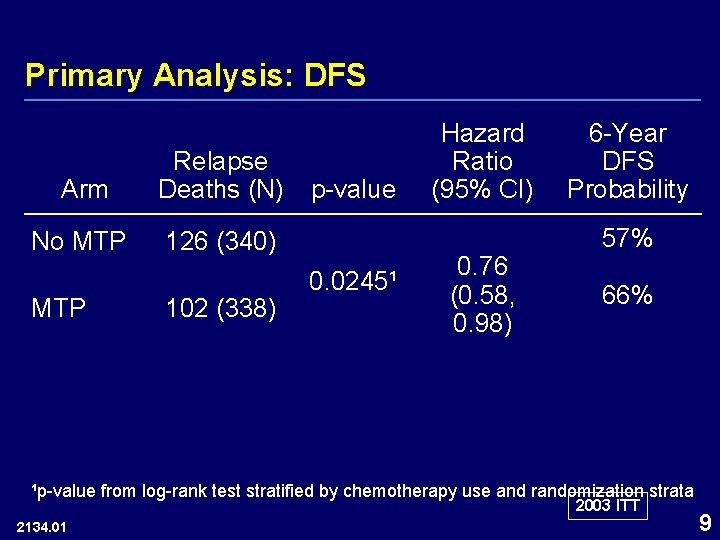
Primary Analysis: DFS Arm Relapse Deaths (N) No MTP 126 (340) MTP 102 (338) p-value 0. 0245¹ Hazard Ratio (95% CI) 0. 76 (0. 58, 0. 98) 6 -Year DFS Probability 57% 66% ¹p-value from log-rank test stratified by chemotherapy use and randomization strata 2003 ITT 2134. 01 9

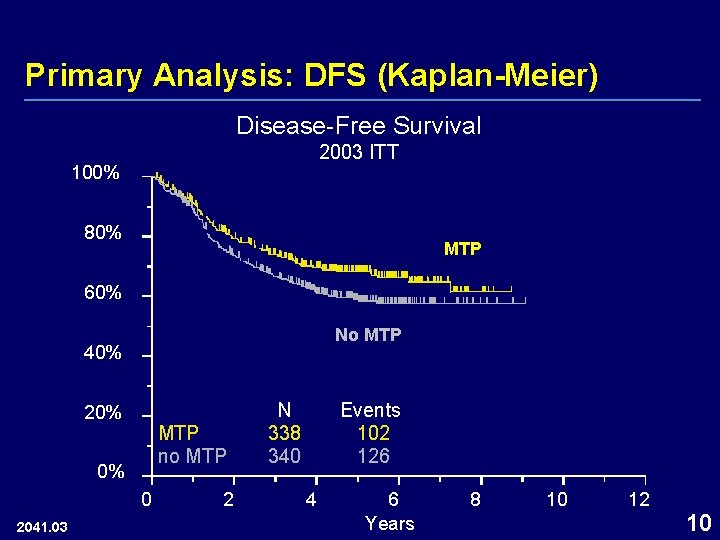
Primary Analysis: DFS (Kaplan-Meier) Disease-Free Survival 2003 ITT 100% 80% MTP 60% No MTP 40% 20% MTP no MTP 0% 0 2041. 03 2 N 338 340 Events 102 126 4 6 Years 8 10 12 10

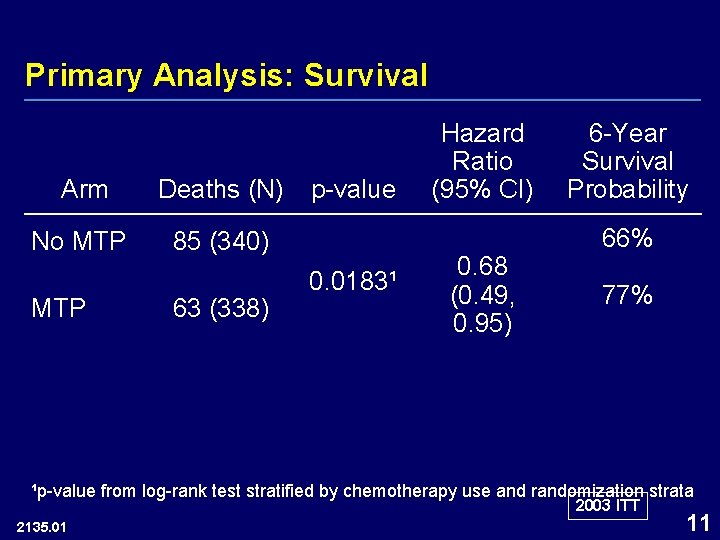
Primary Analysis: Survival Arm Deaths (N) No MTP 85 (340) MTP 63 (338) p-value 0. 0183¹ Hazard Ratio (95% CI) 0. 68 (0. 49, 0. 95) 6 -Year Survival Probability 66% 77% ¹p-value from log-rank test stratified by chemotherapy use and randomization strata 2003 ITT 2135. 01 11

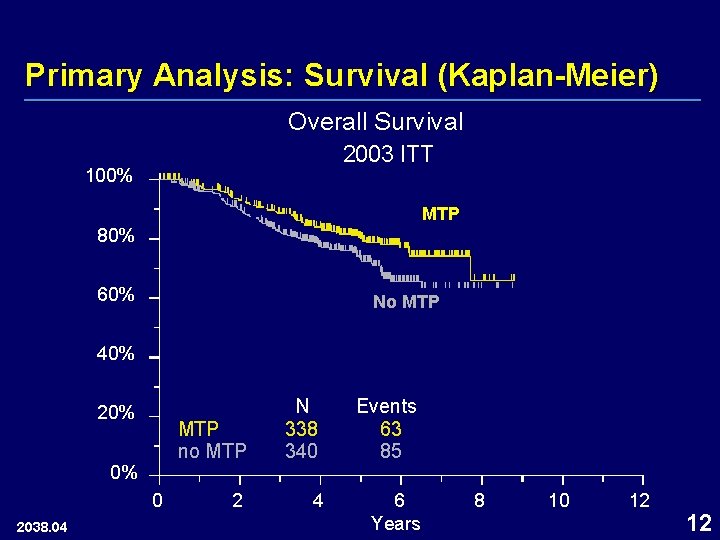
Primary Analysis: Survival (Kaplan-Meier) Overall Survival 2003 ITT 100% MTP 80% 60% No MTP 40% 20% 0% 0 2038. 04 MTP no MTP N 338 340 Events 63 85 2 4 6 Years 8 10 12 12

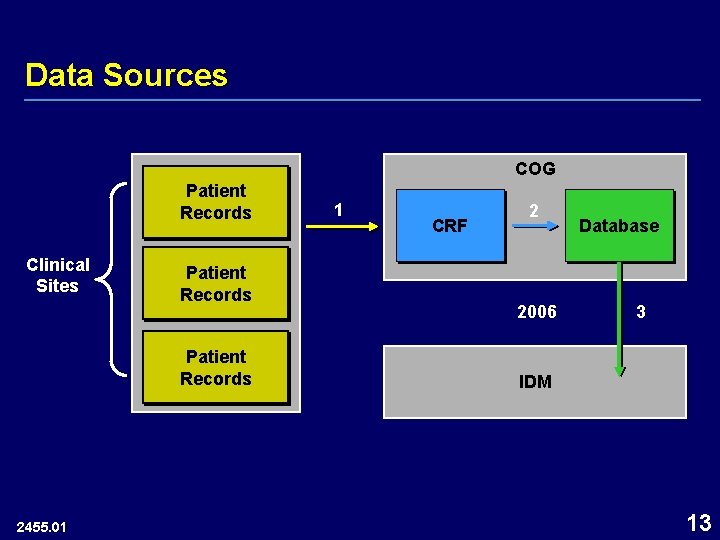
Data Sources COG Patient Records Clinical Sites Patient Records 2455. 01 1 CRF 2 2006 Database 3 IDM 13

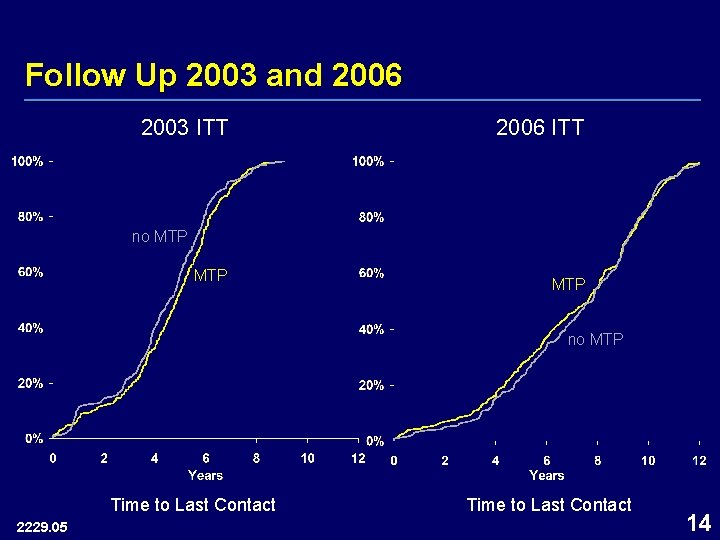
Follow Up 2003 and 2006 2003 ITT 2006 ITT no MTP MTP no MTP Time to Last Contact 2229. 05 Time to Last Contact 14

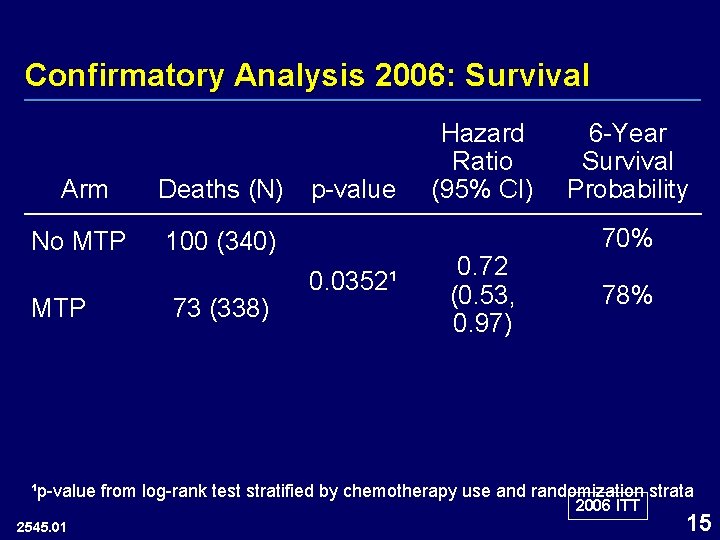
Confirmatory Analysis 2006: Survival Arm Deaths (N) No MTP 100 (340) MTP 73 (338) p-value 0. 0352¹ Hazard Ratio (95% CI) 0. 72 (0. 53, 0. 97) 6 -Year Survival Probability 70% 78% ¹p-value from log-rank test stratified by chemotherapy use and randomization strata 2006 ITT 2545. 01 15

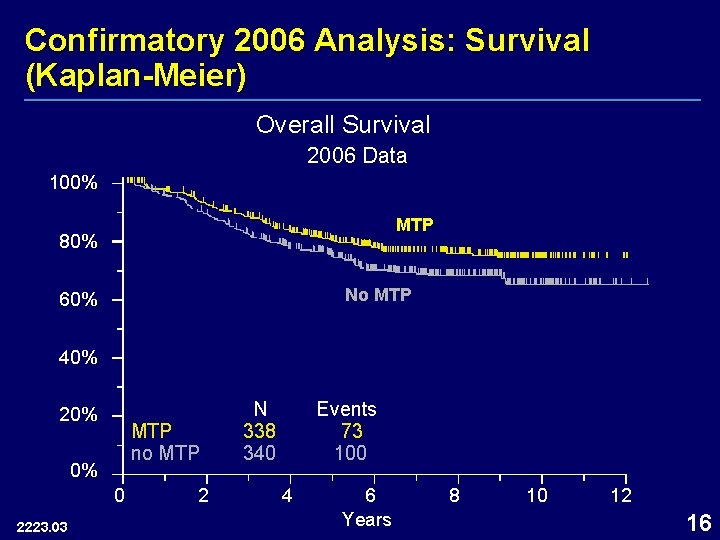
Confirmatory 2006 Analysis: Survival (Kaplan-Meier) Overall Survival 2006 Data 100% MTP 80% No MTP 60% 40% 20% MTP no MTP 0% 0 2223. 03 2 N 338 340 Events 73 100 4 6 Years 8 10 12 16

Safety 2457. 01 17

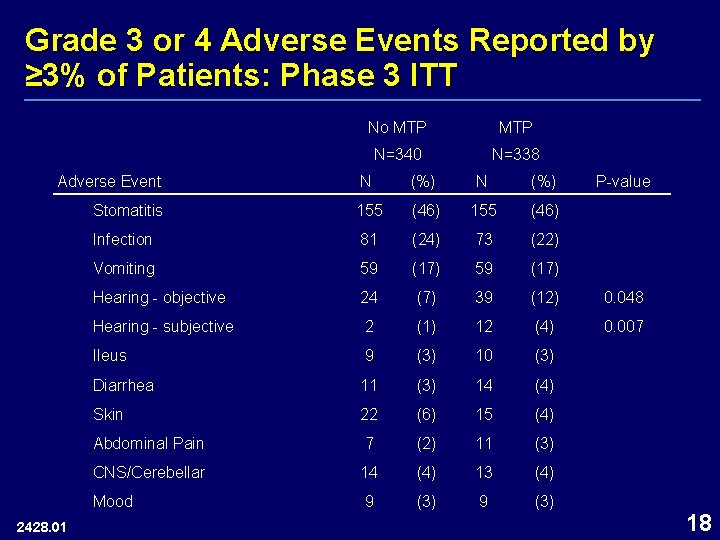
Grade 3 or 4 Adverse Events Reported by ≥ 3% of Patients: Phase 3 ITT Adverse Event 2428. 01 No MTP N=340 N=338 N (%) P-value Stomatitis 155 (46) Infection 81 (24) 73 (22) Vomiting 59 (17) Hearing - objective 24 (7) 39 (12) 0. 048 Hearing - subjective 2 (1) 12 (4) 0. 007 Ileus 9 (3) 10 (3) Diarrhea 11 (3) 14 (4) Skin 22 (6) 15 (4) Abdominal Pain 7 (2) 11 (3) CNS/Cerebellar 14 (4) 13 (4) Mood 9 (3) 18

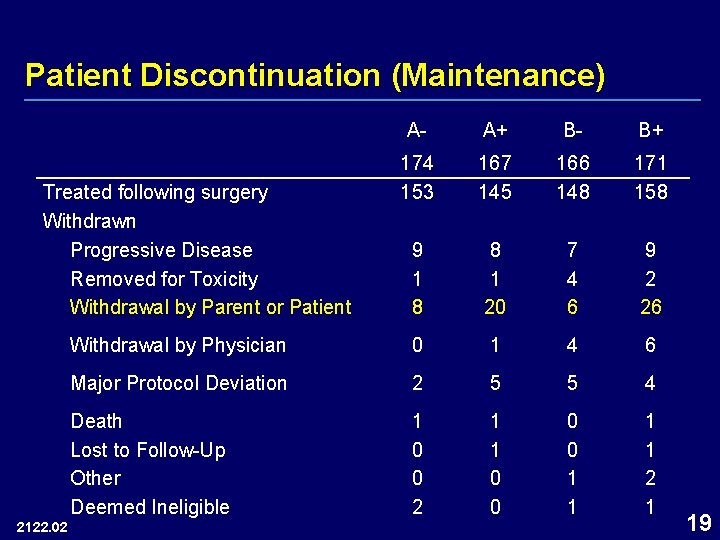
Patient Discontinuation (Maintenance) A- A+ B- B+ 174 153 167 145 166 148 171 158 9 1 8 8 1 20 7 4 6 9 2 26 Withdrawal by Physician 0 1 4 6 Major Protocol Deviation 2 5 5 4 Death Lost to Follow-Up Other Deemed Ineligible 1 0 0 2 1 1 0 0 1 1 2 1 Treated following surgery Withdrawn Progressive Disease Removed for Toxicity Withdrawal by Parent or Patient 2122. 02 19

Osteosarcoma: Overall Survival (SEER Ages 0 - 29) 1993 -2002 1987 -1992 1981 -1986 1975 -1980 2094. 04 20

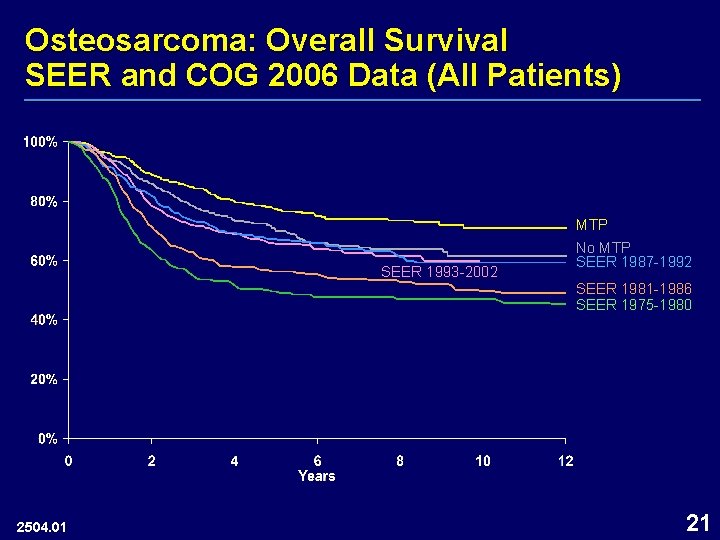
Osteosarcoma: Overall Survival SEER and COG 2006 Data (All Patients) MTP SEER 1993 -2002 No MTP SEER 1987 -1992 SEER 1981 -1986 SEER 1975 -1980 2504. 01 21
 Shawna meyers
Shawna meyers Karin meyers
Karin meyers Mph adhs
Mph adhs Opatus
Opatus Meyers straelen
Meyers straelen E.m. meyers
E.m. meyers Vpm
Vpm Pia meyers md
Pia meyers md Lars kristofferson is the ceo of kristoff markets
Lars kristofferson is the ceo of kristoff markets Nyu natural language processing
Nyu natural language processing Efficacy potency
Efficacy potency Albert bandura self efficacy
Albert bandura self efficacy Efficacy definition pharmacology
Efficacy definition pharmacology Luminous efficacy comparison chart
Luminous efficacy comparison chart Collective teacher efficacy
Collective teacher efficacy Vaccine efficacy
Vaccine efficacy Potency vs efficacy
Potency vs efficacy Drug efficacy
Drug efficacy Collective teacher efficacy
Collective teacher efficacy Types of personality in organisational behaviour
Types of personality in organisational behaviour Vaccine efficacy
Vaccine efficacy Optimal self-confidence
Optimal self-confidence