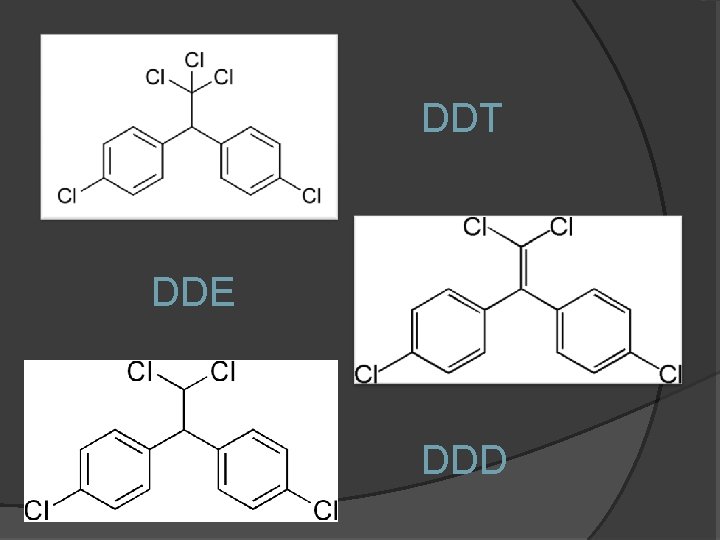
Dwight Causey DDT AND ITS DERIVATIVES DDT DDE







- Slides: 18

Dwight Causey DDT AND ITS DERIVATIVES

DDT DDE DDD

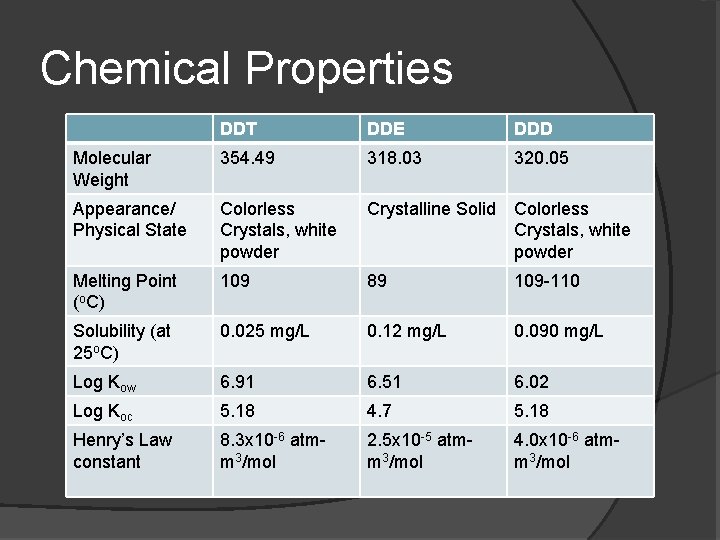
Chemical Properties DDT DDE DDD Molecular Weight 354. 49 318. 03 320. 05 Appearance/ Physical State Colorless Crystals, white powder Crystalline Solid Colorless Crystals, white powder Melting Point (o. C) 109 89 109 -110 Solubility (at 25 o. C) 0. 025 mg/L 0. 12 mg/L 0. 090 mg/L Log Kow 6. 91 6. 51 6. 02 Log Koc 5. 18 4. 7 5. 18 Henry’s Law constant 8. 3 x 10 -6 atmm 3/mol 2. 5 x 10 -5 atmm 3/mol 4. 0 x 10 -6 atmm 3/mol

History First synthesized in 1874 � Insecticidal properties discovered in 1939 by Paul Hermann Müller � � Won Noble Prize in Physiology and Medicine in 1948 Used to control insect-borne diseases (i. e. malaria and typhus) � Peak of usage in 1962 � � Registered for use on 334 agricultural commodities � 85, 000 tons produced � Cumulative estimated world usage is 2 million tons

History Used in homes for mothproofing and lice control Still used today in developing countries to control malaria and lice Silent Spring by Rachel Carson in 1962, questioned the widespread use of DDT


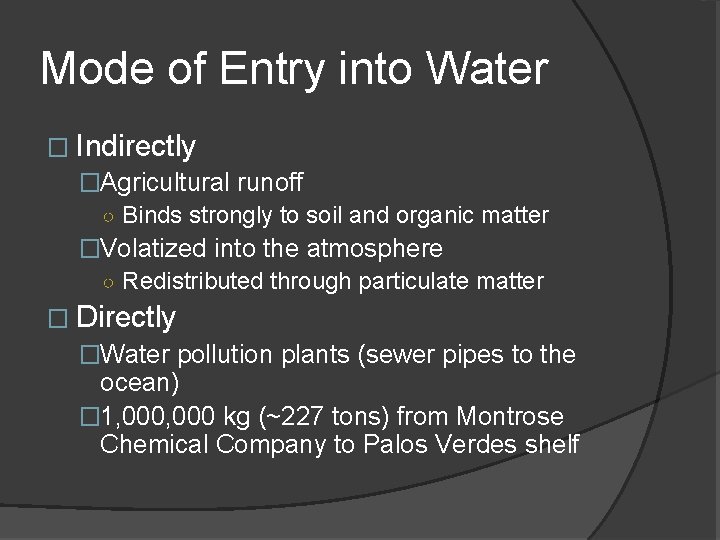
Mode of Entry into Water � Indirectly �Agricultural runoff ○ Binds strongly to soil and organic matter �Volatized into the atmosphere ○ Redistributed through particulate matter � Directly �Water pollution plants (sewer pipes to the ocean) � 1, 000 kg (~227 tons) from Montrose Chemical Company to Palos Verdes shelf

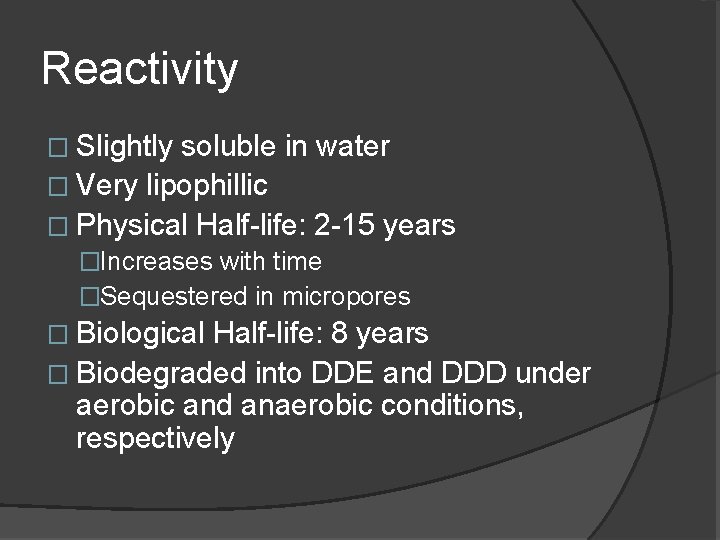
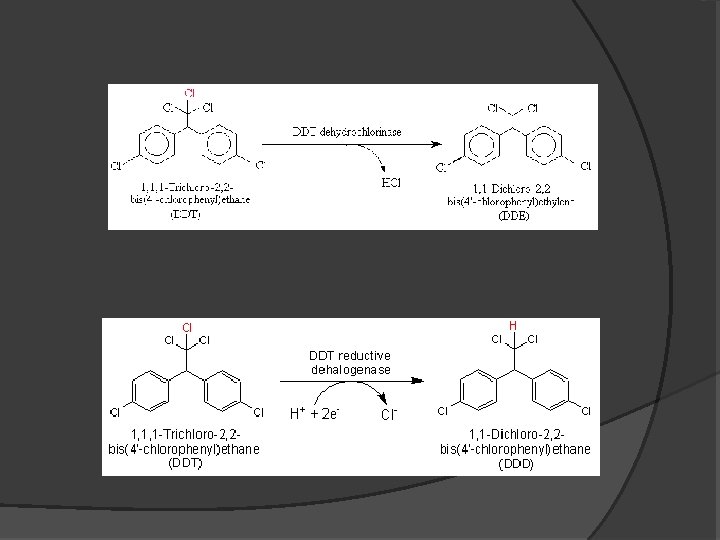
Reactivity � Slightly soluble in water � Very lipophillic � Physical Half-life: 2 -15 years �Increases with time �Sequestered in micropores � Biological Half-life: 8 years � Biodegraded into DDE and DDD under aerobic and anaerobic conditions, respectively


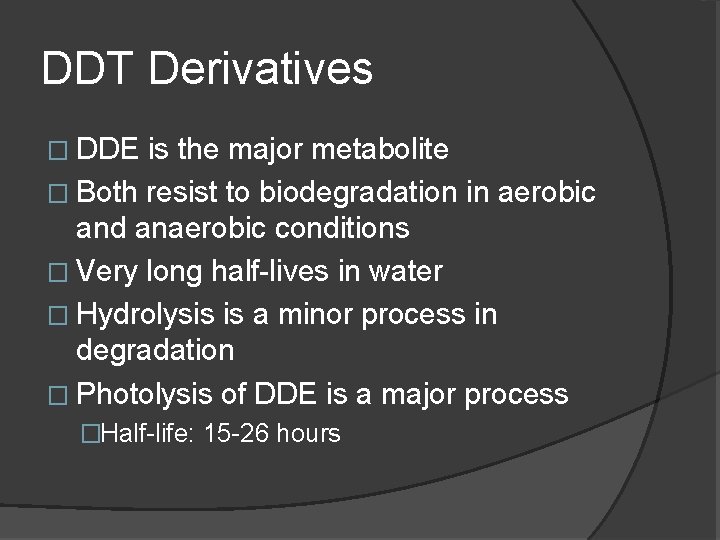
DDT Derivatives � DDE is the major metabolite � Both resist to biodegradation in aerobic and anaerobic conditions � Very long half-lives in water � Hydrolysis is a minor process in degradation � Photolysis of DDE is a major process �Half-life: 15 -26 hours

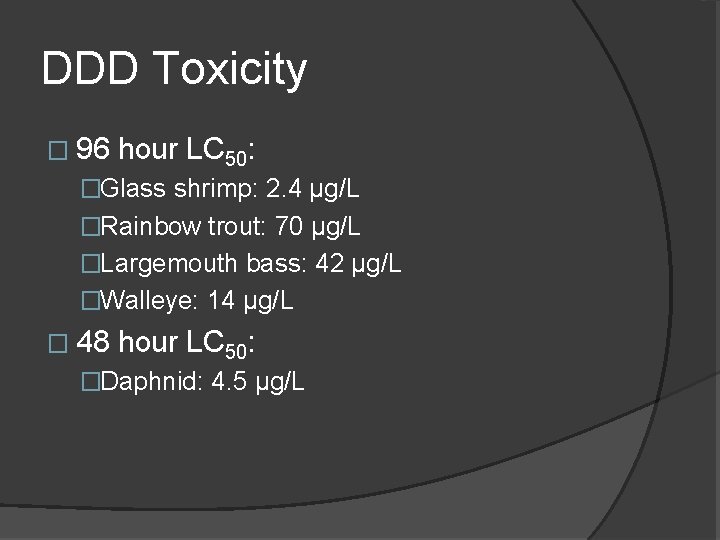
DDD Toxicity � 96 hour LC 50: �Glass shrimp: 2. 4 µg/L �Rainbow trout: 70 µg/L �Largemouth bass: 42 µg/L �Walleye: 14 µg/L � 48 hour LC 50: �Daphnid: 4. 5 µg/L

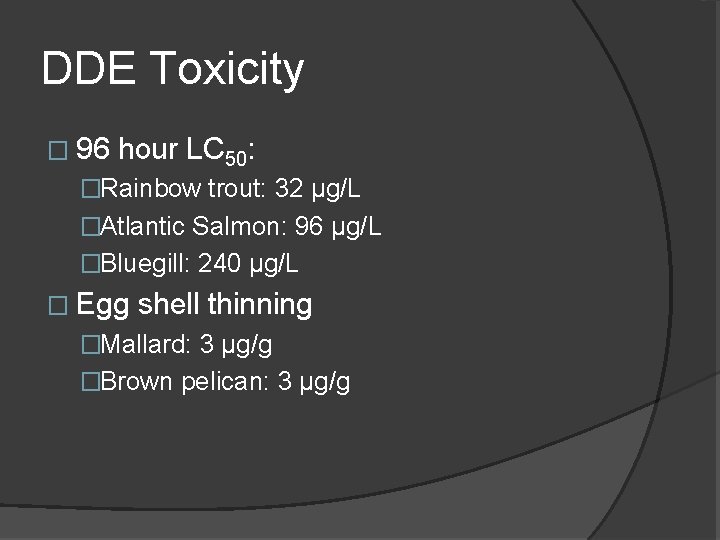
DDE Toxicity � 96 hour LC 50: �Rainbow trout: 32 µg/L �Atlantic Salmon: 96 µg/L �Bluegill: 240 µg/L � Egg shell thinning �Mallard: 3 µg/g �Brown pelican: 3 µg/g

Toxic Effects � Weak estrogenic activities � In the brain: �Inhibition of ATP-based reactions �Hepatic enzyme induction �Disruption of hormonal mechanisms � Inhibition of Na+/K+ ATPases in the gills � Thinning of egg shells in raptors � Reduction in cortisol production

Mode of Entry into Organisms � Majority enters through food � Some enters through absorption from water through body surfaces (i. e. gills), not believed to be significant when compared to amount entering through food � Very Lipophillic, bioaccumulates � Some organisms retain 90%+ of ΣDDT in their food source

Molecular Mode of Interaction � Egg shell thinning in Raptors, 2 possibilities: � DDE inhibits prostaglandin synthesis in the shell gland mucosa, limiting calcium and bicarbonate transport across the mucosa � Embryonic exposure alters shell gland carbonic anhydrase expression, causing eggshell thinning � In fish, no known molecular mechanism is known � Believed to involve ATPases in the central nervous system and gills � In Insects, causes the irreversible opening of voltage gated Na+ channels along the axon

Biochemical Metabolism and Breakdown DDT metabolized into DDE and DDD by microorganisms � Mixed-function oxidases may induce the dechlorination of DDT to DDE in fishes � In some mammals, DDE is converted to 2 methylsulfonyl-DDE and 3 -methylsulfonyl. DDE � �Acted on by phase I CYP 2 B enzymes �Followed by conjugation with glutathione during phase II �Then through the mercapturic acid pathway, 2 SH-DDE and 3 -SH-DDE are formed

Detoxification Up-regulation of CYP 6 G 1 gene in Drosophila melanogaster � Secretion through urine, feces, semen, and breast milk � Clams shown to dechlorinate DDE to DDMU under methanogenic or sulfidogenic conditions � Dried and ground seaweed has been shown to increase DDT biodegradation by 80% after 6 weeks, further degradation of DDD also seen �

Bibliography � � � Cal/Ecotox Toxicity Data for Brown Pelican (Pelecanus occidentalis). Office of Environmental Health Hazard Assessment. 1999. http: //www. oehha. ca. gov/cal_ecotox/report/pelectox. pdf The Comparative Toxicogenomics Database. Mount Desert Island Biological Laboratory. 2008. http: //ctd. mdibl. org/ Denholm I, Devine GJ, Williamson MS (2002). Evolutionary genetics. Insecticide resistance on the move. Science 297 (5590): 2222– 3. Evans, D. H. (1987). The Fish Gill: Site of Action and Model for Toxic Effects of Environmental Pollutants. Environmental Health Perspectives 71, 47 -58. Hazardous Substances Data Bank. National Library of Medicine TOXNET System. 2008. http: //toxmap. nlm. nih. gov/toxmap/home/welcome. do Handbook of Acute Toxicity of Chemicals to Fish and Aquatic Invertebrates. U. S. Fish and Wildlife Services. 1980. Kantachote D. , Naidu R. , Williams B. , Mc. Clure N. , Megharaj M. , Singleton I. (2004). Bioremediation of DDTcontaminated soil: enhancement by seaweed addition. Journal of Chemical Technology & Biotechnology, 79 , 6, 632 -638. Lacroix M. , Hontela A. (2003). The organochlorine o, p’-DDD disrupts the adrenal steroidogenic signaling pathway in rainbow trout (Oncorhynchus mykiss). Toxicology and Applied Pharmacology 190, 197 -205. O’Reilly A. O. , Khambay B. P. S. , Williamson M. S. , Field L. M. , Wallace B. A. , Emyr Davies T. G. (2006). Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochemical Journal, 396, 255 -263. U. S. Department of Health and Human Services. Toxicological Profile for DDT, DDE, and DDD. Agency for Toxic Substances and Disease Registry. 2002. World Health Organization. DDT and its Derivatives – Environmental Aspects. Environmental Health Criteria 83. 1989. http: //www. inchem. org/documents/ehc/ehc 83. htm World Health Organization. DDT and its Derivatives. Environmental Health Criteria 9. 1979. http: //www. inchem. org/documents/ehc/ehc 009. htm