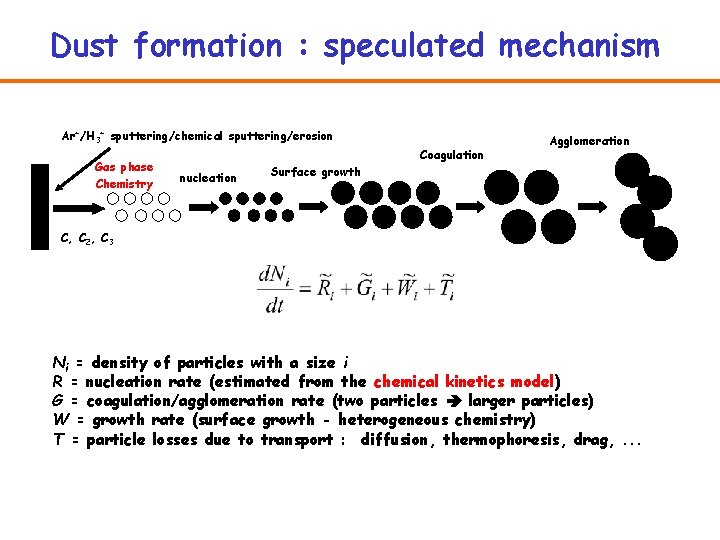
Dust formation speculated mechanism ArH 3 sputteringchemical sputteringerosion

- Slides: 20

Dust formation : speculated mechanism Ar+/H 3+ sputtering/chemical sputtering/erosion Gas phase Chemistry Coagulation nucleation Agglomeration Surface growth C, C 2, C 3 Ni = density of particles with a size i R = nucleation rate (estimated from the chemical kinetics model) G = coagulation/agglomeration rate (two particles larger particles) W = growth rate (surface growth - heterogeneous chemistry) T = particle losses due to transport : diffusion, thermophoresis, drag, . . .

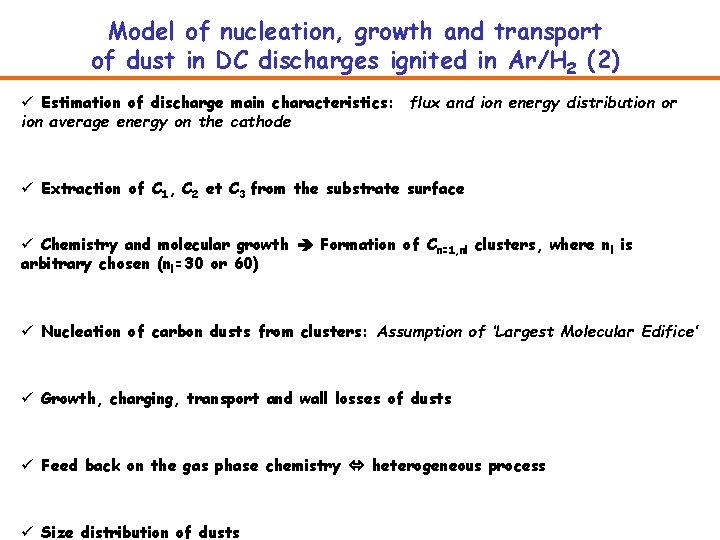
Model of nucleation, growth and transport of dust in DC discharges ignited in Ar/H 2 (2) ü Estimation of discharge main characteristics: flux and ion energy distribution or ion average energy on the cathode ü Extraction of C 1, C 2 et C 3 from the substrate surface ü Chemistry and molecular growth Formation of Cn=1, nl clusters, where nl is arbitrary chosen (nl=30 or 60) ü Nucleation of carbon dusts from clusters: Assumption of ‘Largest Molecular Edifice’ ü Growth, charging, transport and wall losses of dusts ü Feed back on the gas phase chemistry heterogeneous process ü Size distribution of dusts

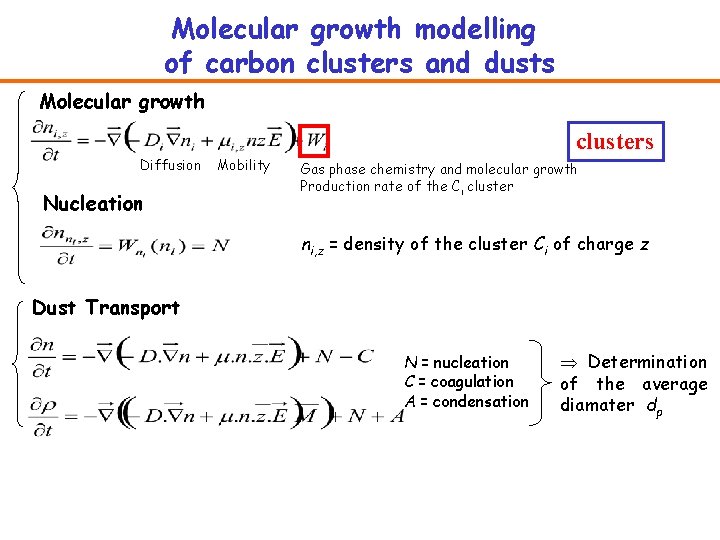
Molecular growth modelling of carbon clusters and dusts Molecular growth clusters Diffusion Nucleation Mobility Gas phase chemistry and molecular growth Production rate of the Ci cluster ni, z = density of the cluster Ci of charge z Dust Transport N = nucleation C = coagulation A = condensation Þ Determination of the average diamater dp

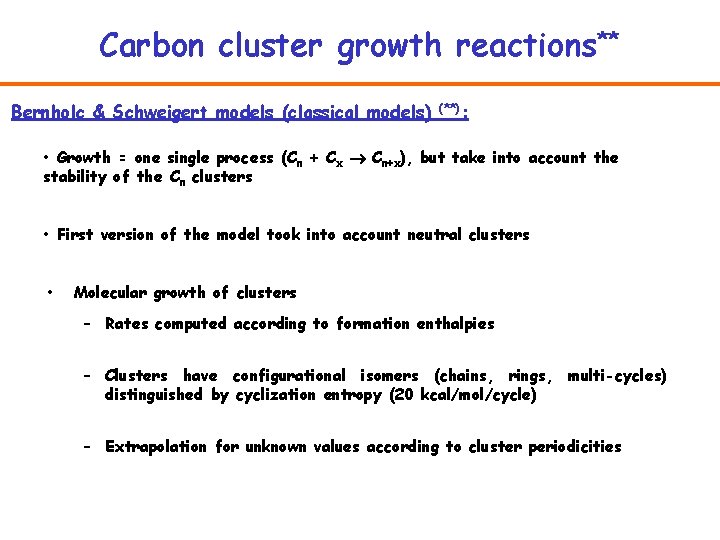
Carbon cluster growth reactions** Bernholc & Schweigert models (classical models) (**): • Growth = one single process (Cn + Cx Cn+x), but take into account the stability of the Cn clusters • First version of the model took into account neutral clusters • Molecular growth of clusters – Rates computed according to formation enthalpies – Clusters have configurational isomers (chains, rings, multi-cycles) distinguished by cyclization entropy (20 kcal/mol/cycle) – Extrapolation for unknown values according to cluster periodicities

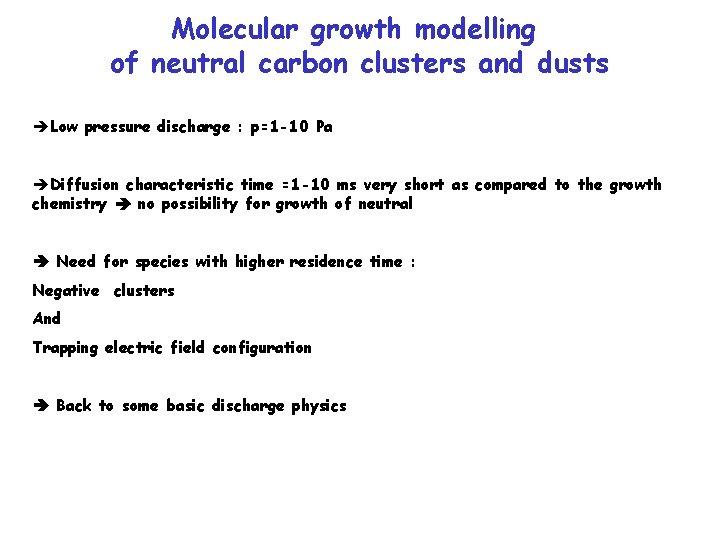
Molecular growth modelling of neutral carbon clusters and dusts Low pressure discharge : p=1 -10 Pa Diffusion characteristic time =1 -10 ms very short as compared to the growth chemistry no possibility for growth of neutral Need for species with higher residence time : Negative clusters And Trapping electric field configuration Back to some basic discharge physics

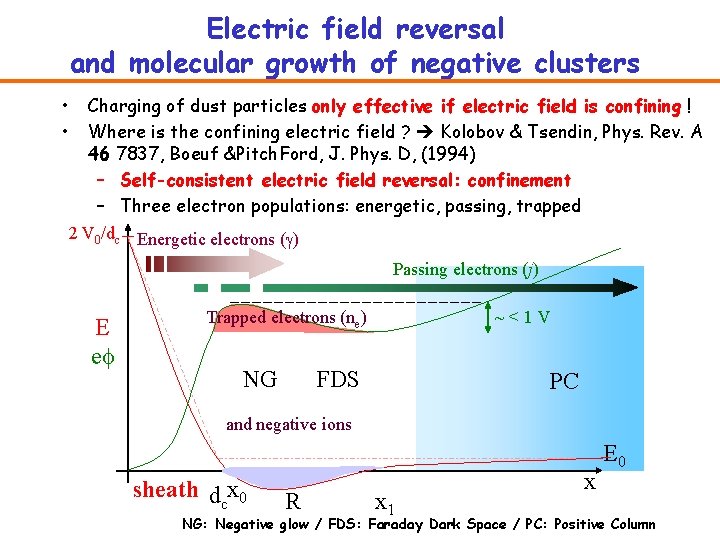
Electric field reversal and molecular growth of negative clusters • • Charging of dust particles only effective if electric field is confining ! Where is the confining electric field ? Kolobov & Tsendin, Phys. Rev. A 46 7837, Boeuf &Pitch. Ford, J. Phys. D, (1994) – Self-consistent electric field reversal: confinement – Three electron populations: energetic, passing, trapped 2 V 0/dc Energetic electrons (g) Passing electrons (j) E ef ~<1 V Trapped electrons (ne) NG FDS PC and negative ions sheath dcx 0 R x 1 x E 0 NG: Negative glow / FDS: Faraday Dark Space / PC: Positive Column

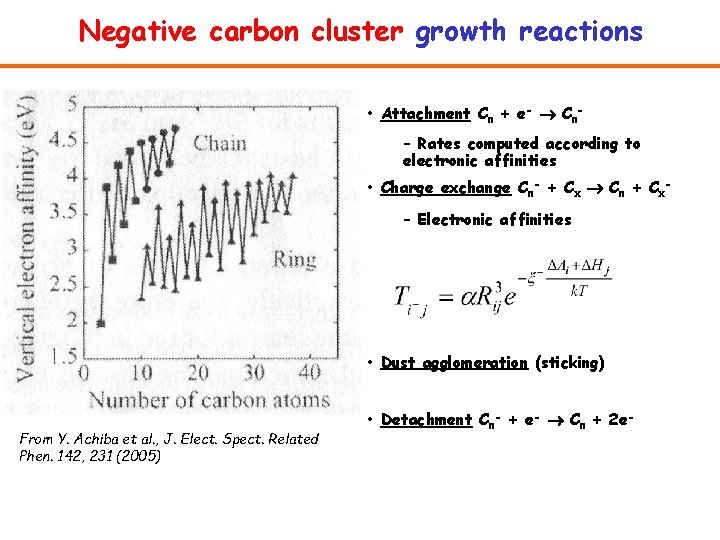
Negative carbon cluster growth reactions • Attachment Cn + e- Cn– Rates computed according to electronic affinities • Charge exchange Cn- + Cx Cn + Cx– Electronic affinities • Dust agglomeration (sticking) From Y. Achiba et al. , J. Elect. Spect. Related Phen. 142, 231 (2005) • Detachment Cn- + e- Cn + 2 e-

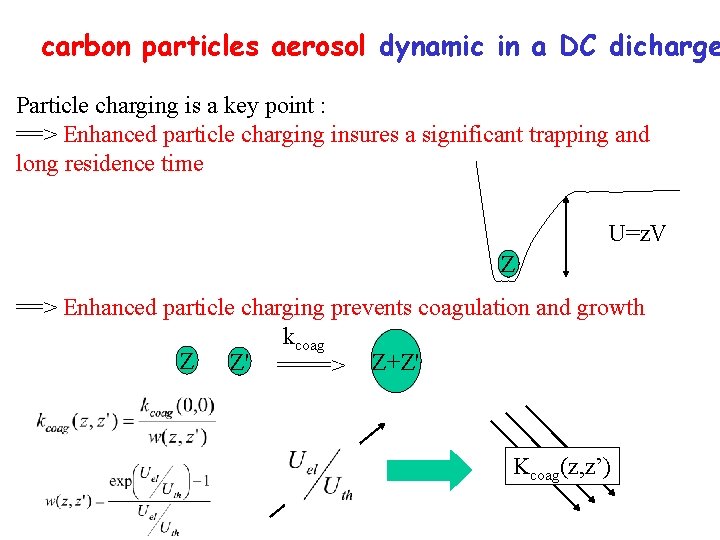
carbon particles aerosol dynamic in a DC dicharge Particle charging is a key point : ==> Enhanced particle charging insures a significant trapping and long residence time U=z. V Z ==> Enhanced particle charging prevents coagulation and growth kcoag Z Z' ====> Z+Z' Kcoag(z, z’)

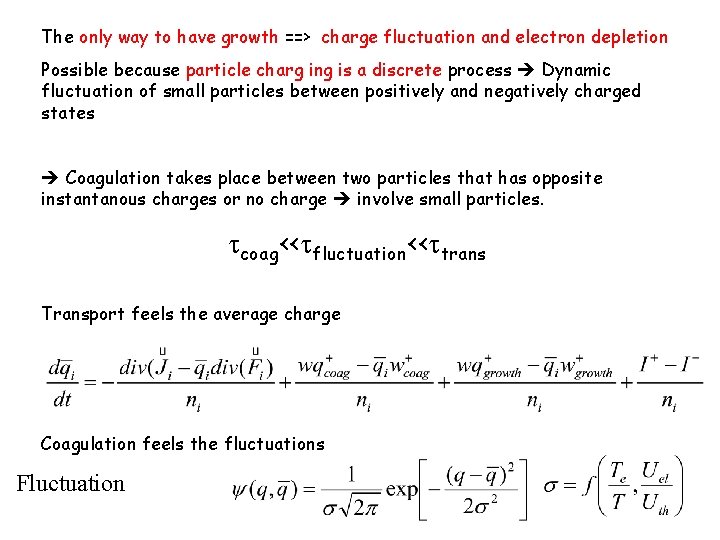
The only way to have growth ==> charge fluctuation and electron depletion Possible because particle charg ing is a discrete process Dynamic fluctuation of small particles between positively and negatively charged states Coagulation takes place between two particles that has opposite instantanous charges or no charge involve small particles. tcoag<<tfluctuation<<ttrans Transport feels the average charge Coagulation feels the fluctuations Fluctuation

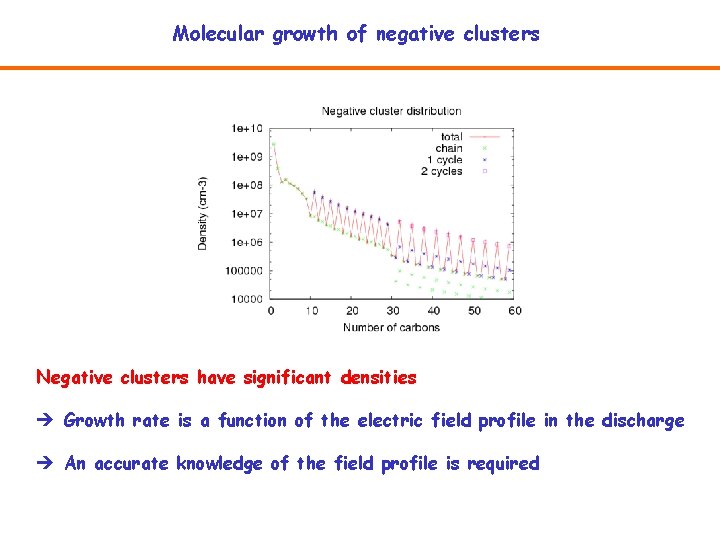
Molecular growth of negative clusters Negative clusters have significant densities Growth rate is a function of the electric field profile in the discharge An accurate knowledge of the field profile is required

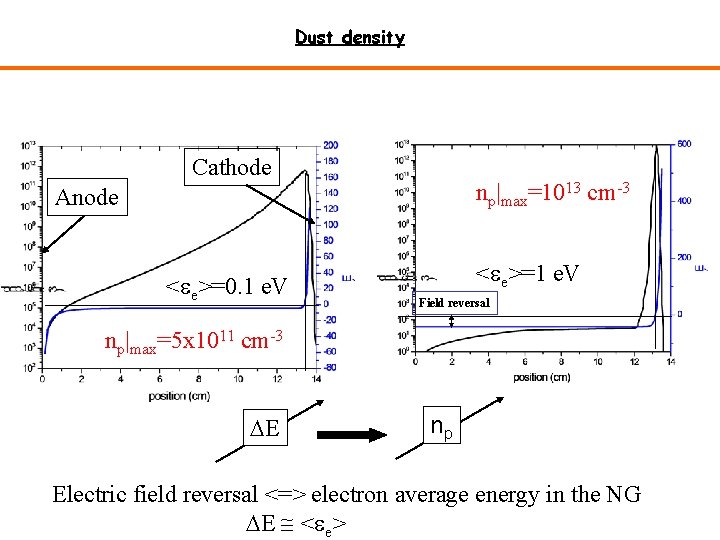
Dust density Cathode np|max=1013 cm-3 Anode <ee>=0. 1 e. V <ee>=1 e. V Field reversal np|max=5 x 1011 cm-3 DE np Electric field reversal <=> electron average energy in the NG DE <ee>

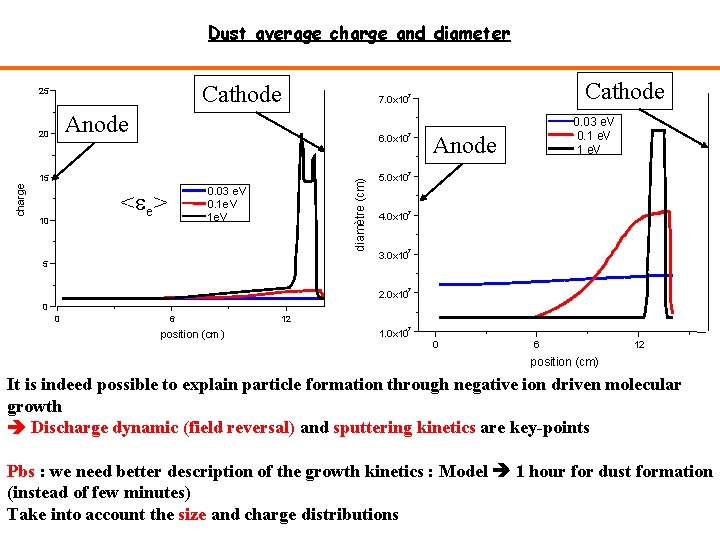
Dust average charge and diameter Cathode 25 Anode 20 -7 6. 0 x 10 0. 03 e. V 0. 1 e. V Anode -7 0. 03 e. V 0. 1 e. V <ee> 10 diamètre (cm) 15 charge Cathode -7 7. 0 x 10 5 5. 0 x 10 -7 4. 0 x 10 -7 3. 0 x 10 -7 2. 0 x 10 0 0 6 position (cm) 12 -7 1. 0 x 10 0 6 12 position (cm) It is indeed possible to explain particle formation through negative ion driven molecular growth Discharge dynamic (field reversal) and sputtering kinetics are key-points Pbs : we need better description of the growth kinetics : Model 1 hour for dust formation (instead of few minutes) Take into account the size and charge distributions

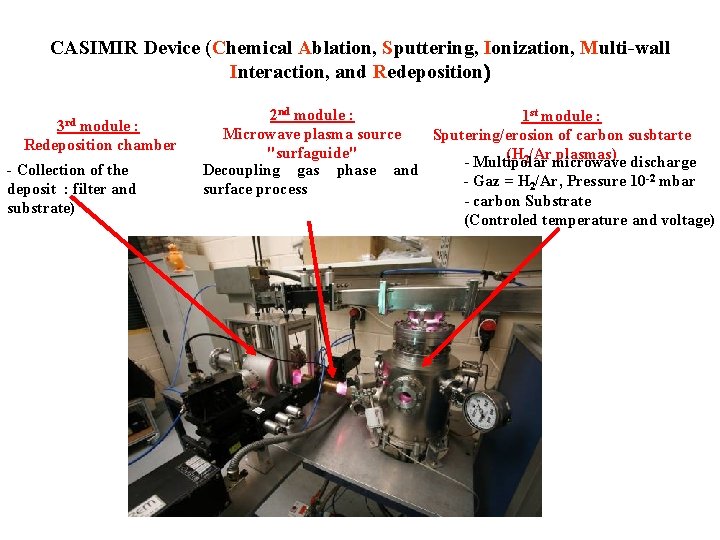
CASIMIR Device (Chemical Ablation, Sputtering, Ionization, Multi-wall Interaction, and Redeposition) 3 rd module : Redeposition chamber - Collection of the deposit : filter and substrate) 2 nd module : 1 st module : Microwave plasma source Sputering/erosion of carbon susbtarte "surfaguide" (H 2/Armicrowave plasmas) discharge Multipolar Decoupling gas phase and - Gaz = H 2/Ar, Pressure 10 -2 mbar surface process - carbon Substrate (Controled temperature and voltage)

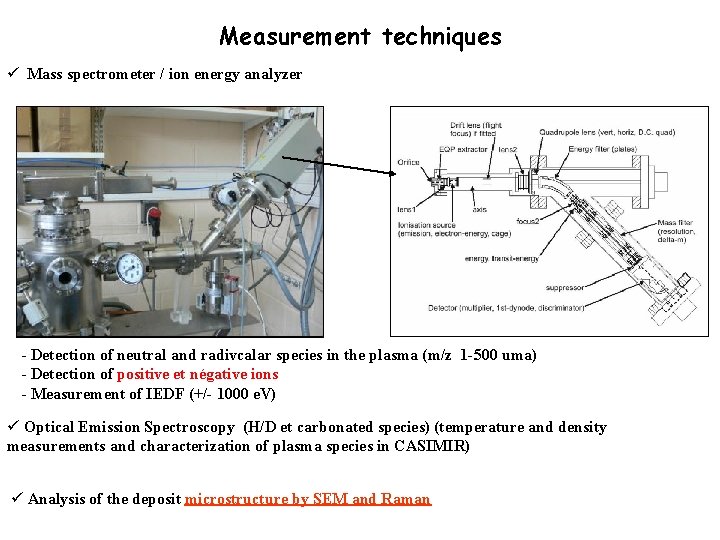
Measurement techniques ü Mass spectrometer / ion energy analyzer - Detection of neutral and radivcalar species in the plasma (m/z 1 -500 uma) - Detection of positive et négative ions - Measurement of IEDF (+/- 1000 e. V) ü Optical Emission Spectroscopy (H/D et carbonated species) (temperature and density measurements and characterization of plasma species in CASIMIR) ü Analysis of the deposit microstructure by SEM and Raman

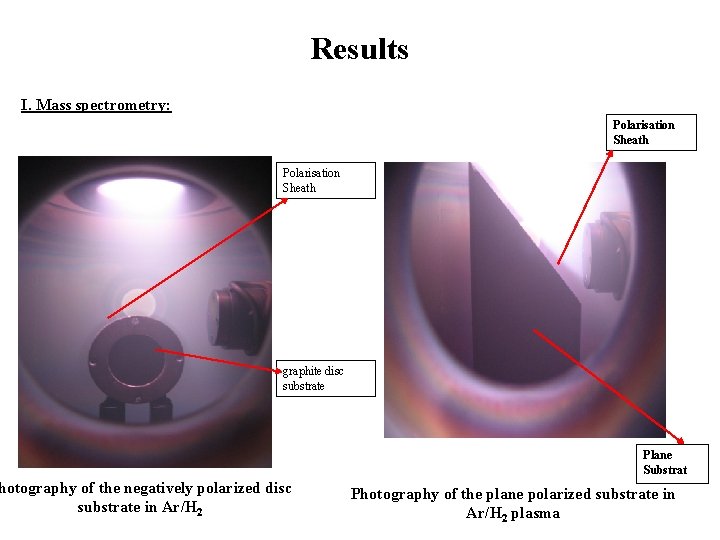
Results I. Mass spectrometry: Polarisation Sheath graphite disc substrate hotography of the negatively polarized disc substrate in Ar/H 2 Plane Substrat Photography of the plane polarized substrate in Ar/H 2 plasma

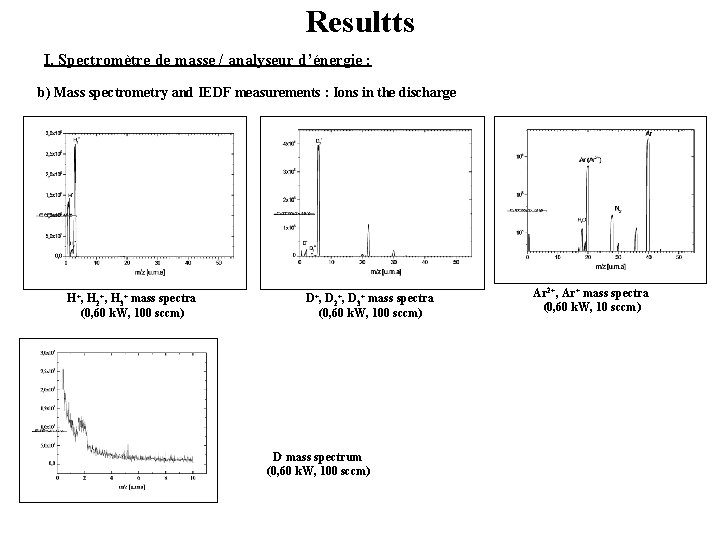
Resultts I. Spectromètre de masse / analyseur d’énergie : b) Mass spectrometry and IEDF measurements : Ions in the discharge H+, H 2+, H 3+ mass spectra (0, 60 k. W, 100 sccm) D+, D 2+, D 3+ mass spectra (0, 60 k. W, 100 sccm) D- mass spectrum (0, 60 k. W, 100 sccm) Ar 2+, Ar+ mass spectra (0, 60 k. W, 10 sccm)

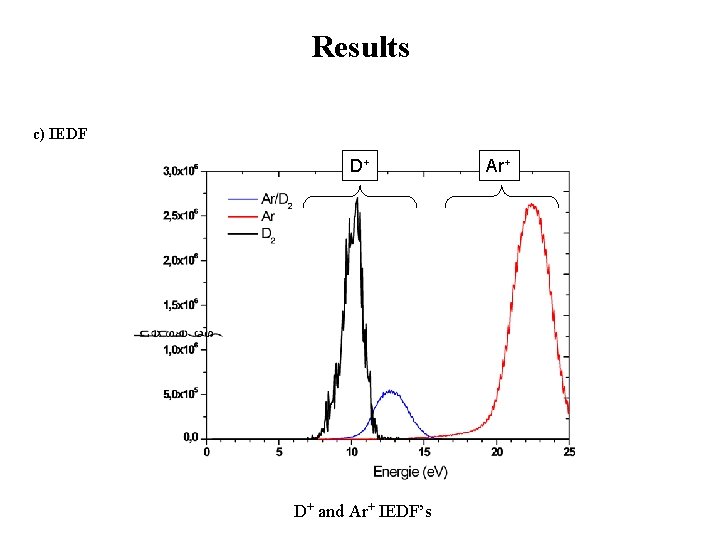
Results c) IEDF D+ D+ and Ar+ IEDF’s Ar+

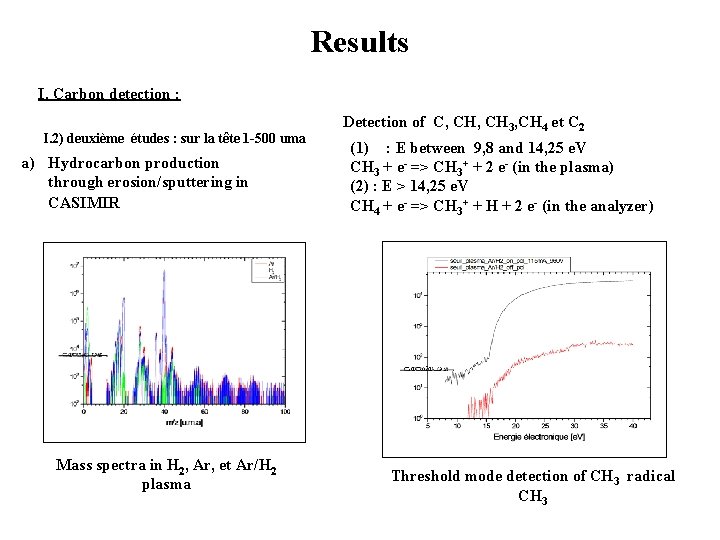
Results I. Carbon detection : I. 2) deuxième études : sur la tête 1 -500 uma a) Hydrocarbon production through erosion/sputtering in CASIMIR Mass spectra in H 2, Ar, et Ar/H 2 plasma Detection of C, CH 3, CH 4 et C 2 (1) : E between 9, 8 and 14, 25 e. V CH 3 + e- => CH 3+ + 2 e- (in the plasma) (2) : E > 14, 25 e. V CH 4 + e- => CH 3+ + H + 2 e- (in the analyzer) Threshold mode detection of CH 3 radical CH 3

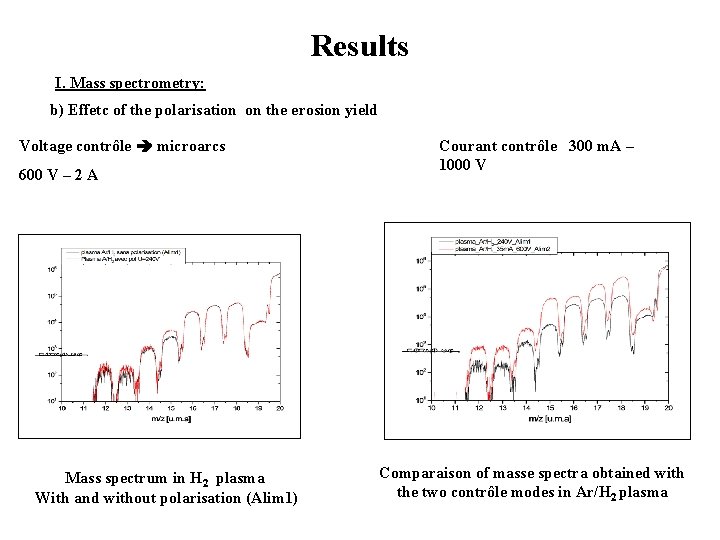
Results I. Mass spectrometry: b) Effetc of the polarisation on the erosion yield Voltage contrôle microarcs 600 V – 2 A Mass spectrum in H 2 plasma With and without polarisation (Alim 1) Courant contrôle 300 m. A – 1000 V Comparaison of masse spectra obtained with the two contrôle modes in Ar/H 2 plasma

 Moodle arh bg
Moodle arh bg Arh
Arh Arh citrix
Arh citrix Fawaz aldabbagh
Fawaz aldabbagh Barnum effect
Barnum effect Reaction formation
Reaction formation Formation initiale vs formation continue
Formation initiale vs formation continue Kris pister
Kris pister Pixie wps
Pixie wps Dust bowl
Dust bowl Smart dust
Smart dust Dust bowl slideshow
Dust bowl slideshow Used to lay out any noncircular curve
Used to lay out any noncircular curve Chalkdustdiva
Chalkdustdiva Wrap fugitive dust handbook
Wrap fugitive dust handbook Snow by robert frost
Snow by robert frost The dust code
The dust code Dust bowl map
Dust bowl map Pictures of the dust bowl
Pictures of the dust bowl Diseases caused by dust
Diseases caused by dust Lattice degeneration
Lattice degeneration