DRCR Retina Network Vitreous Proteomics in Eyes with











- Slides: 18

DRCR Retina Network Vitreous Proteomics in Eyes with a Macular Hole (Protocol AJ) Protocol Chair: Sharon Solomon, MD

Study Rationale Ø There is an ongoing need to better understand key molecular pathways in the pathogenesis of retinal diseases. Ø Elucidation of proteins involved in these pathways could: § Improve our scientific understanding § Identify novel targets for more effective treatment § Enhance our ability to predict disease course or response to currently available treatments Ø Vitreous is a potentially rich source of proteins that may directly reflect biochemical processes that are active in or influence the retina.

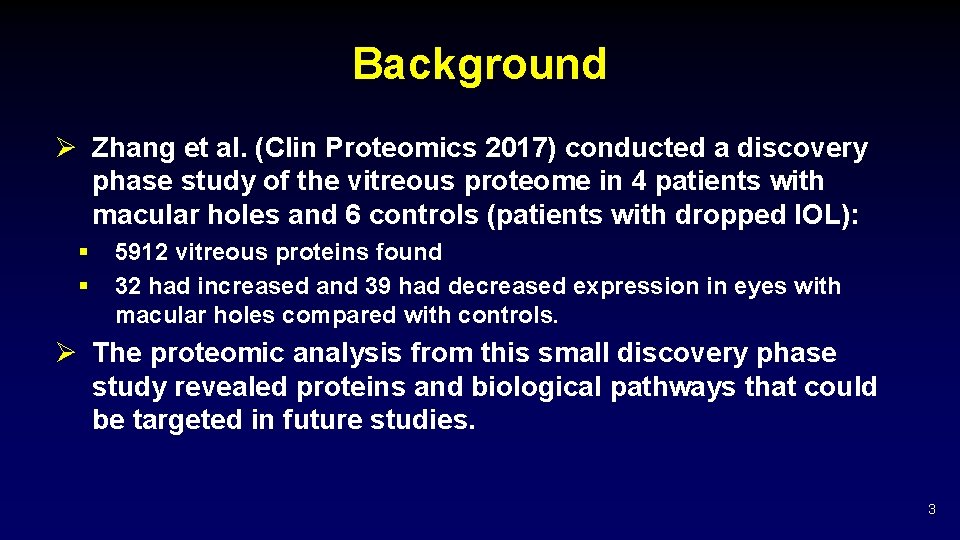
Background Ø Zhang et al. (Clin Proteomics 2017) conducted a discovery phase study of the vitreous proteome in 4 patients with macular holes and 6 controls (patients with dropped IOL): § § 5912 vitreous proteins found 32 had increased and 39 had decreased expression in eyes with macular holes compared with controls. Ø The proteomic analysis from this small discovery phase study revealed proteins and biological pathways that could be targeted in future studies. 3

Study Objective Ø Verify and characterize abnormally expressed vitreous proteins in adults with full thickness macular holes (MH). Ø Identify pathways involved in the pathogenesis of macular hole formation to determine potential targets for therapeutic intervention. 4

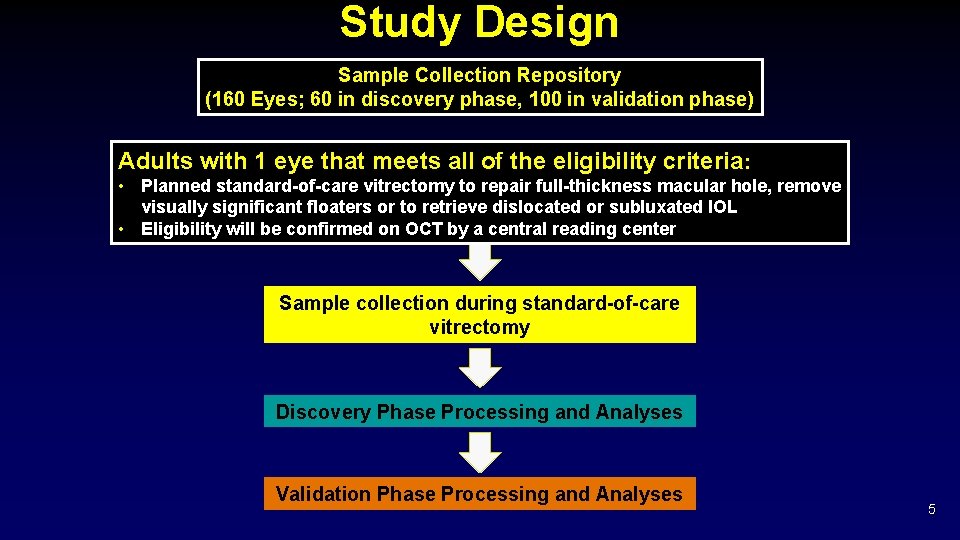
Study Design Sample Collection Repository (160 Eyes; 60 in discovery phase, 100 in validation phase) Adults with 1 eye that meets all of the eligibility criteria: • Planned standard-of-care vitrectomy to repair full-thickness macular hole, remove visually significant floaters or to retrieve dislocated or subluxated IOL • Eligibility will be confirmed on OCT by a central reading center Sample collection during standard-of-care vitrectomy Discovery Phase Processing and Analyses Validation Phase Processing and Analyses 5

Discovery Phase Ø Sample size: 60 eyes § 20 eyes with MH § 20 eyes with dislocated IOL § 20 eyes with visually significant floaters Ø Determine which proteins are up- and down-regulated in the vitreous of eyes with MH. Ø Processing and analyses include: § Tandem mass tag (TMT) labeling § Processing the samples and analyzing with mass spectrometry § Bioinformatics 6

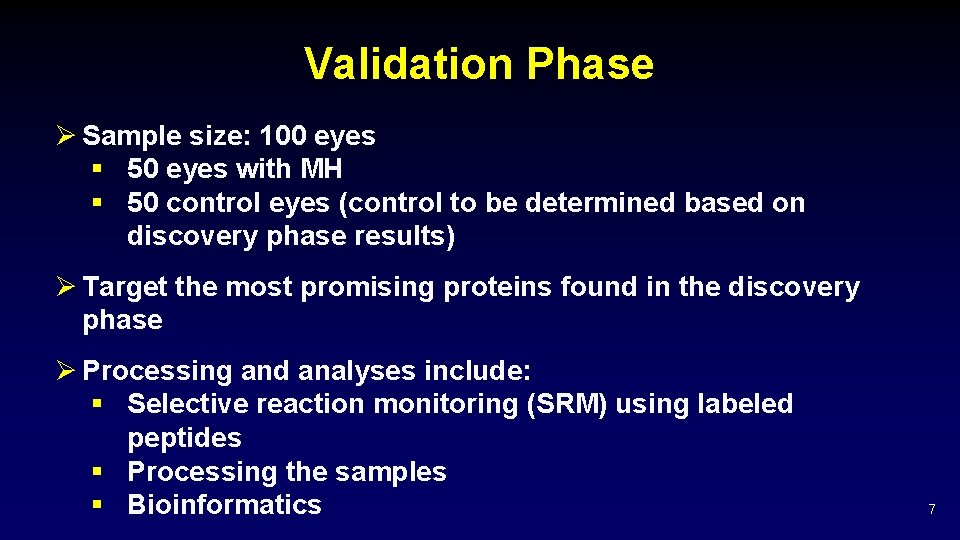
Validation Phase Ø Sample size: 100 eyes § 50 eyes with MH § 50 control eyes (control to be determined based on discovery phase results) Ø Target the most promising proteins found in the discovery phase Ø Processing and analyses include: § Selective reaction monitoring (SRM) using labeled peptides § Processing the samples § Bioinformatics 7

Major Eligibility Criteria Ø ≥ 18 years old Ø No type 1 or type 2 diabetes Ø Study Eye Criteria § Undergoing standard of care vitrectomy for one of the following: • Repair full thickness macular hole • Retrieve dislocated or subluxated IOL • Remove vitreous opacities causing floaters 8

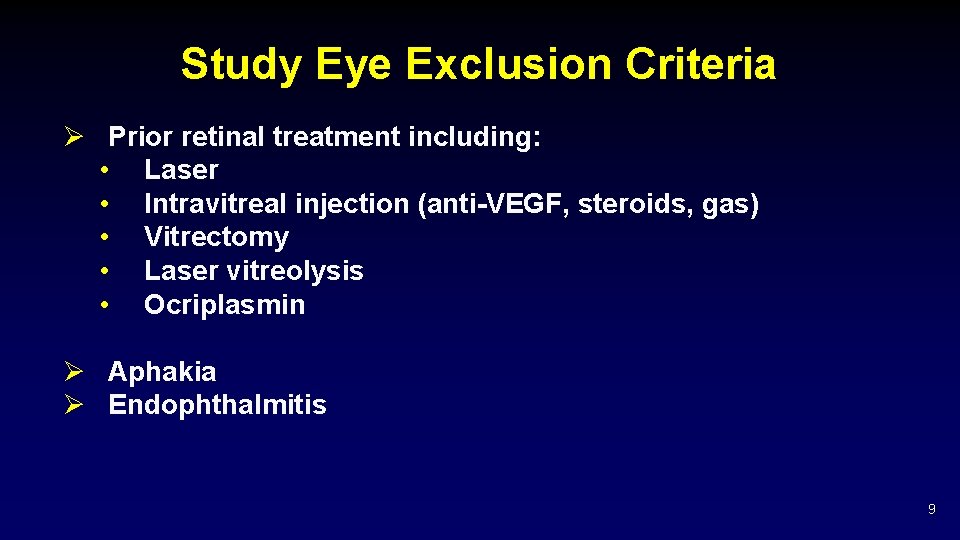
Study Eye Exclusion Criteria Ø Prior retinal treatment including: • Laser • Intravitreal injection (anti-VEGF, steroids, gas) • Vitrectomy • Laser vitreolysis • Ocriplasmin Ø Aphakia Ø Endophthalmitis 9

Study Eye Exclusion Criteria Ø Any retinal abnormalities (macular hole with ERM or VMT) is not an exclusion) • Vitreous hemorrhage, AMD, secondary macular edema • History of vein occlusion • History of retinal detachment Ø Other ocular abnormalities including • Uveitis or history of uveitis • Recent trauma • History of high myopia or high level of myopia § Defined as spherical equivalent of -8. 00 diopters or more myopic if phakic or exam evidence of high myopia if phakic or pseudophakic 10

Procedures Ø Study consists of a single visit to assess eligibility and collect clinic data § All procedures are standard care and must provide enough information to complete the online case report forms Screening Procedures Clinic Visual Acuity Clinic SD OCT (Cirrus or Spectralis) Clinic Ocular Exam Medical and Ocular History 11

Procedures Ø All procedures must be completed within 1 month prior to vitrectomy Ø If vitrectomy is moved more than one month from the screening procedures, eligibility may be impacted. Please contact the coordinating center if surgery is moved. 12

Study Procedures Ø The clinic OCT must be uploaded promptly for a central reading center to confirm eligibility. § It is strongly recommended that the OCT scans are as follows to ensure eligibility can be assessed: • Minimum 49 -line dense volume macula scan (20° x 20° on Spectralis, 512 x 128 on Cirrus) • High resolution scans (7 -line high res raster scan on Spectralis, 5 -line HD raster scan on Cirrus) Ø You will only hear if the eye is NOT eligible; please proceed with sample collection during vitrectomy unless the coordinating center contacts you. 13

Sample Collection Procedure Ø See the Study Procedure Manual for full details Ø The following should be ready or available on the day of collection: § Sample Collection Kit (including shipper, triplicate barcodes, and Fed. Ex label) § Two copies of blank Vitreous Sample Transmittal Log § Dry ice available (or -80°C freezer will be used) 14

Sample Collection Procedure Ø Prior to sample collection, the barcode label is placed by the coordinator or physician on the collection tube. Ø The duplicate labels should also be affixed to the two copies of the transmittal log. § Include one copy with the sample shipment § Use the other copy for data entry on the study website • Please complete website data entry on the same day as surgery, as this alerts the lab that a sample has been shipped 15

Sample Collection Procedure Ø At least 1 cc undiluted vitreous (or as much as the surgeon is comfortable taking) should be obtained with a closed infusion line by manual aspiration with cutting on into a syringe connected along the aspiration line Ø Aliquot the sample into the labeled 1. 5 cc microcentrfuge tube with a screw top Ø Freeze the sample immediately (within 15 minutes) at -80° C or place on dry ice immediately for shipment to the central lab. 16

Sample Shipping Reminders Ø The central lab does not accept and process weekend delivery of samples until the following Monday. Please schedule the vitrectomy surgery Monday-Thursday and ship the same day so that viable samples are received at the lab on weekdays. Ø Samples must be shipped in accordance to IATA regulations. Please ensure that IATA or dry ice trained staff person is available on the scheduled surgery date or the sample can be stored immediately in a -80° C freezer until the certified staff is available to ship the sample. Ø It is important to use the shipper provided. 17

Thank You 18
 Drcr retina network
Drcr retina network Drcr.net protocol
Drcr.net protocol Drcr.net protocol summary
Drcr.net protocol summary Protocol i drcr
Protocol i drcr Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Ranibizumab
Ranibizumab Mini trans-blot module
Mini trans-blot module Yosin hitomi
Yosin hitomi History of proteomics
History of proteomics Comparative proteomics kit ii western blot module
Comparative proteomics kit ii western blot module Seismic analysis code download
Seismic analysis code download Comparative proteomics kit ii western blot module
Comparative proteomics kit ii western blot module Prionproteine
Prionproteine Texture of sheep eye lens
Texture of sheep eye lens Cow eye optic disc
Cow eye optic disc Mr lett
Mr lett Muscae volitantes
Muscae volitantes Optic disc cow eye
Optic disc cow eye Hessah vessel
Hessah vessel