Dimensional Analysis USING DIMENSIONS TO v CONVERT UNITS







- Slides: 14

Dimensional Analysis USING DIMENSIONS TO v. CONVERT UNITS v. SOLVE PROBLEMS Neely's Chemistry

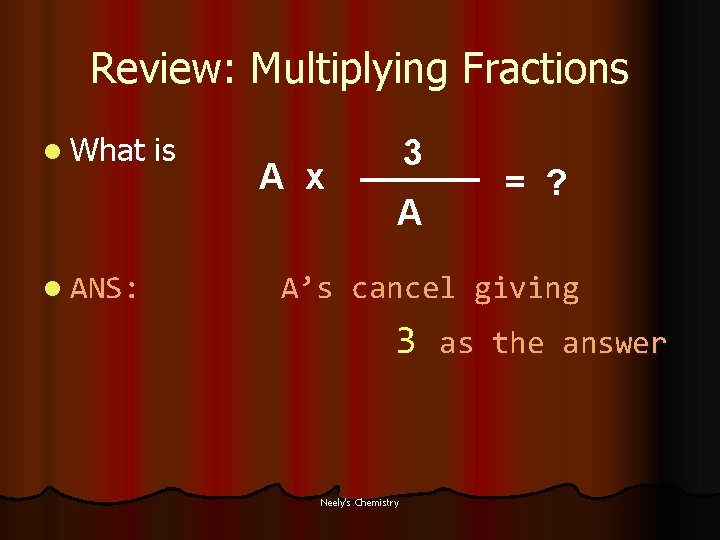
Review: Multiplying Fractions l What l ANS: is A x 3 A = ? A’s cancel giving 3 Neely's Chemistry as the answer

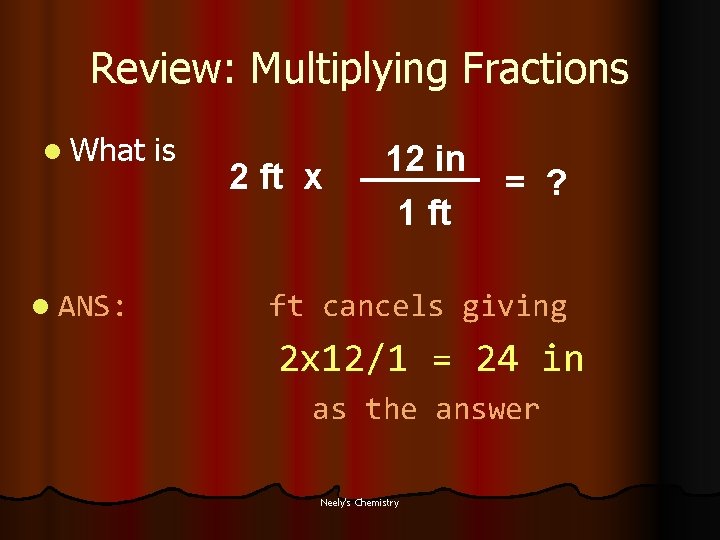
Review: Multiplying Fractions l What l ANS: is 2 ft x 12 in 1 ft = ? ft cancels giving 2 x 12/1 = 24 in as the answer Neely's Chemistry

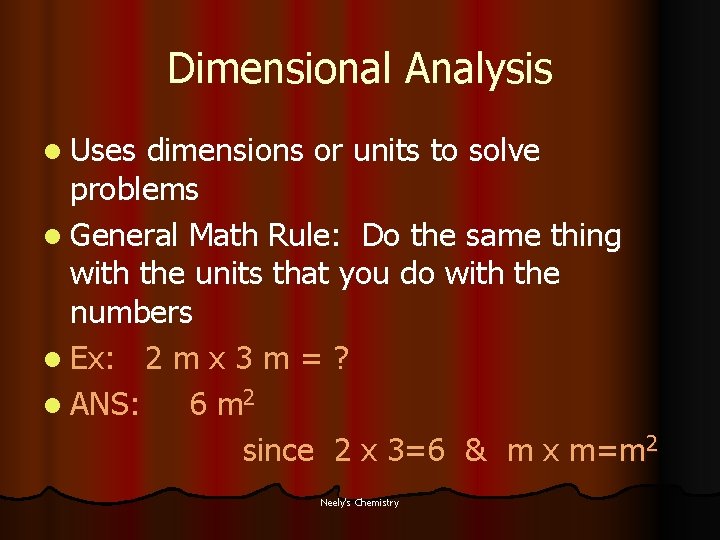
Dimensional Analysis l Uses dimensions or units to solve problems l General Math Rule: Do the same thing with the units that you do with the numbers l Ex: 2 m x 3 m = ? l ANS: 6 m 2 since 2 x 3=6 & m x m=m 2 Neely's Chemistry

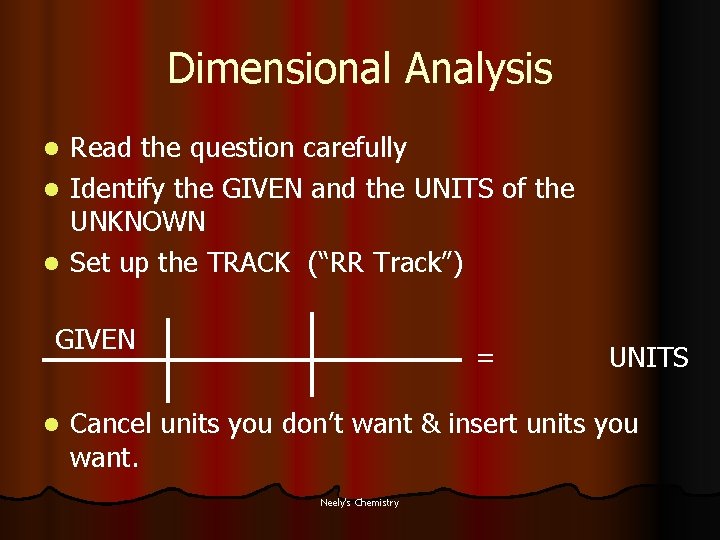
Dimensional Analysis l Read the question carefully l Identify the GIVEN and the UNITS of the UNKNOWN Neely's Chemistry

Dimensional Analysis Read the question carefully l Identify the GIVEN and the UNITS of the UNKNOWN l Set up the TRACK (“RR Track”) l GIVEN l = UNITS Cancel units you don’t want & insert units you want. Neely's Chemistry

How many yards is 54 inches? Neely's Chemistry

How many yards is 56 inches? l l l GIVEN = 54 in UNITS for unknown = yd Track: 54 in l l l 1 ft 1 yd = 1. 5 yd 12 in 3 ft Don’t want inches in final answer What do you know that will get the desired units? 1 ft = 12 in & 3 ft = 1 yd Neely's Chemistry

l Congratulations! Now you have solved a unit conversion problem by dimensional analysis! Neely's Chemistry

Let’s Solve a Word Problem l The density of mercury is 13. 5 g/m. L. What volume of mercury in L would weigh 2700 g? Neely's Chemistry

l The density of mercury is 13. 5 g/m. L. What volume of mercury in L would weigh 2700 g? 2700 g 1 m. L 1 L 13. 5 g 1000 m. L l Congratulations! = 0. 20 L Now you have solved a word problem by dimensional analysis! Neely's Chemistry

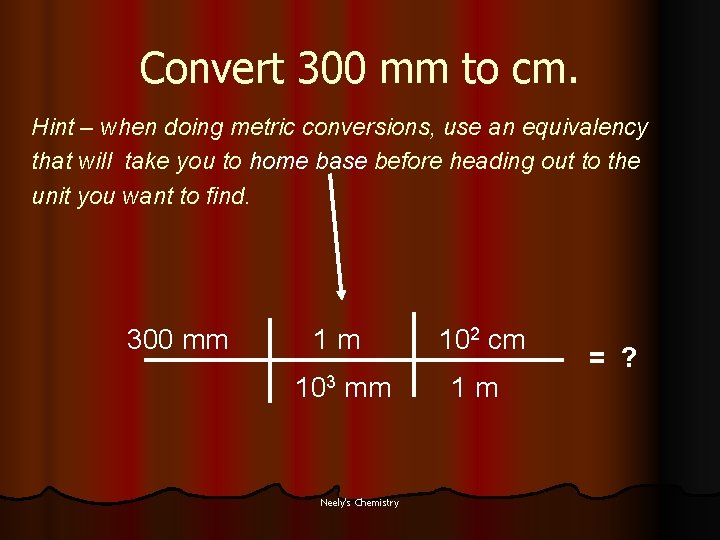
Convert 300 mm to cm. Hint – when doing metric conversions, use an equivalency that will take you to home base before heading out to the unit you want to find. 300 mm 1 m 102 cm 103 mm 1 m Neely's Chemistry = ?

PRACTICE : o How many hours in a year? o The Baconater from Wendy’s has 8 strips of bacon. How many strips of bacon are there in 16 Baconaters? o It is 5954 m from KHS to Hwy 59. How many miles is that? (There are 1. 61 km in a mile. ) Neely's Chemistry

Time to work on the Dimensional Analysis Practice WS Neely's Chemistry