Dimensional analysis and Units of Measurements Dimensional analysis































- Slides: 68

Dimensional analysis and Units of Measurements

Dimensional analysis • Dimensional analysis uses conversion factors to convert from one unit to another. • Also called Factor Label (and railroad tracks) • You do this in your head all the time – How many quarters are in 4 dollars?
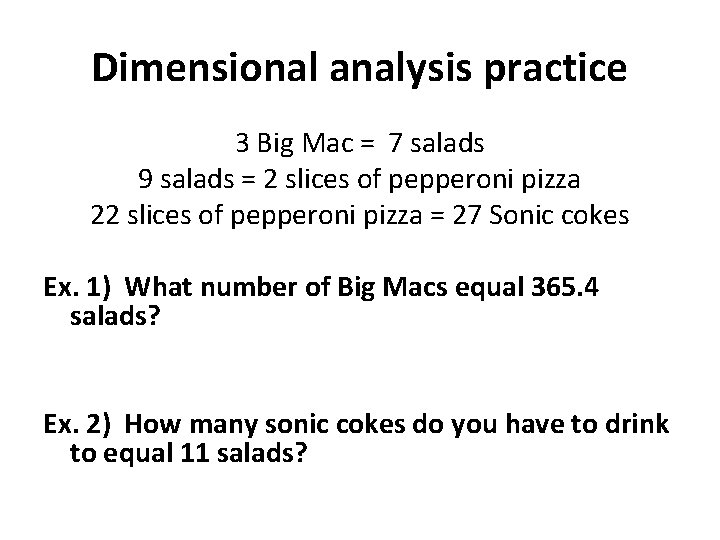
Dimensional analysis practice 3 Big Mac = 7 salads 9 salads = 2 slices of pepperoni pizza 22 slices of pepperoni pizza = 27 Sonic cokes Ex. 1) What number of Big Macs equal 365. 4 salads? Ex. 2) How many sonic cokes do you have to drink to equal 11 salads?

Units of Measurement Meter m Liter L Celsius C

Mass is the amount of matter, weight is a measure of the gravitational pull on matter

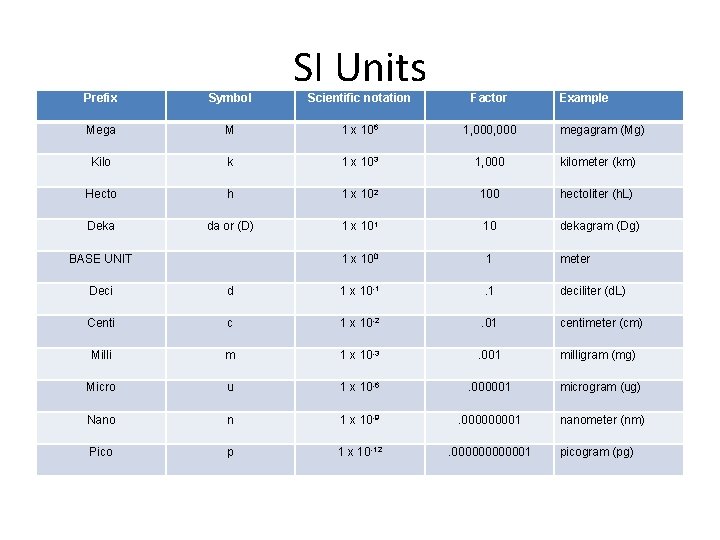
Prefix Symbol Mega SI Units Scientific notation Factor M 1 x 106 1, 000 Kilo k 1 x 103 1, 000 kilometer (km) Hecto h 1 x 102 100 hectoliter (h. L) Deka da or (D) 1 x 101 10 dekagram (Dg) 1 x 100 1 meter BASE UNIT Example megagram (Mg) Deci d 1 x 10 -1 . 1 deciliter (d. L) Centi c 1 x 10 -2 . 01 centimeter (cm) Milli m 1 x 10 -3 . 001 milligram (mg) Micro u 1 x 10 -6 . 000001 microgram (ug) Nano n 1 x 10 -9 . 00001 nanometer (nm) Pico p 1 x 10 -12 . 0000001 picogram (pg)

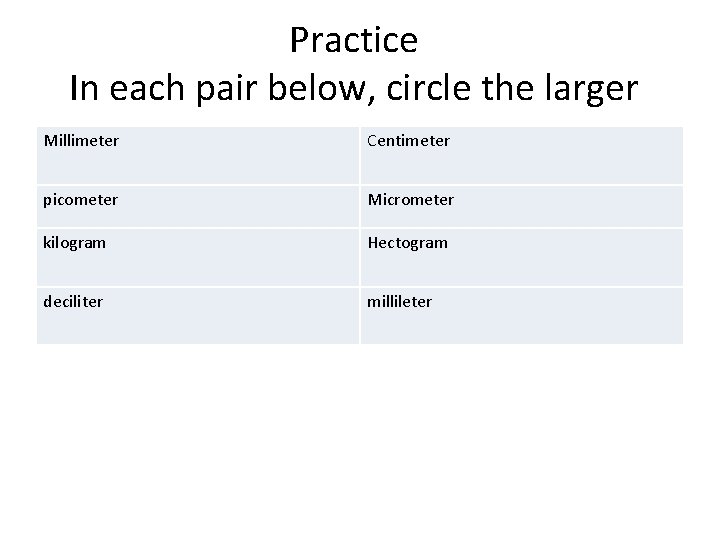
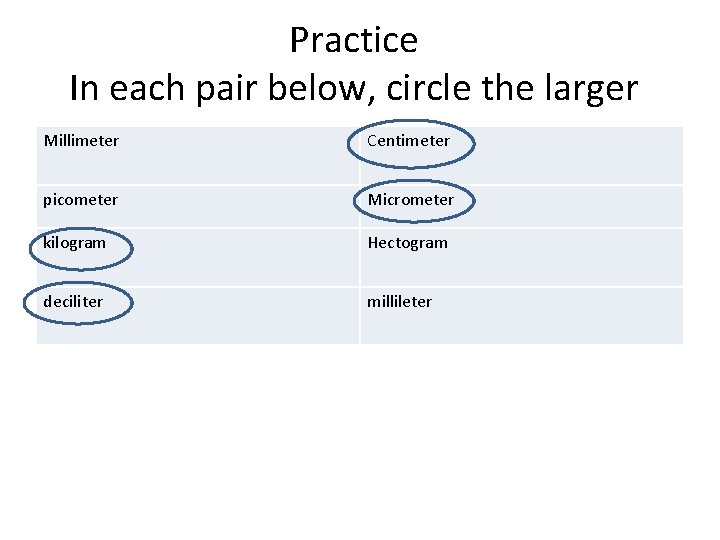
Practice In each pair below, circle the larger Millimeter Centimeter picometer Micrometer kilogram Hectogram deciliter millileter

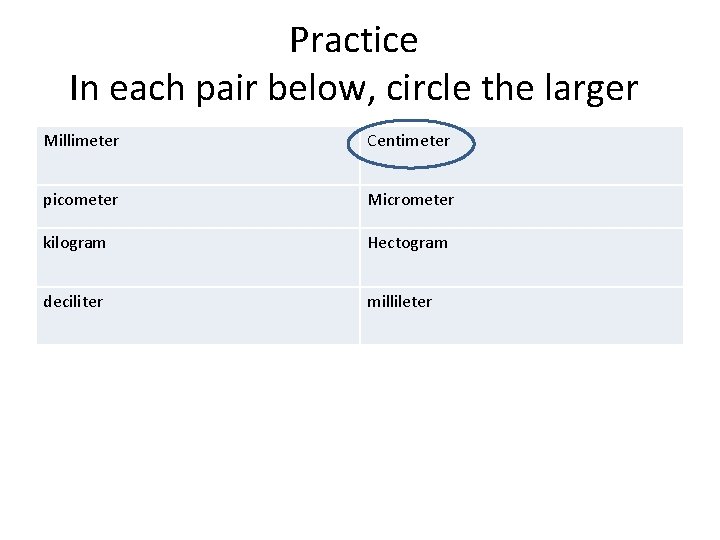
Practice In each pair below, circle the larger Millimeter Centimeter picometer Micrometer kilogram Hectogram deciliter millileter

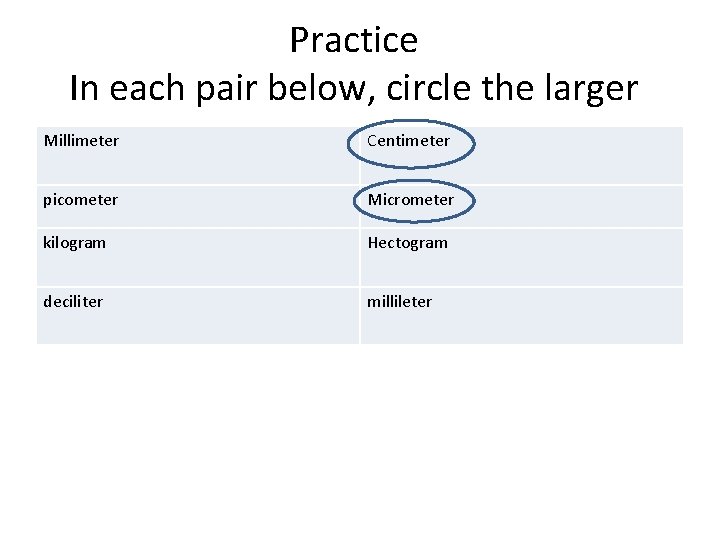
Practice In each pair below, circle the larger Millimeter Centimeter picometer Micrometer kilogram Hectogram deciliter millileter

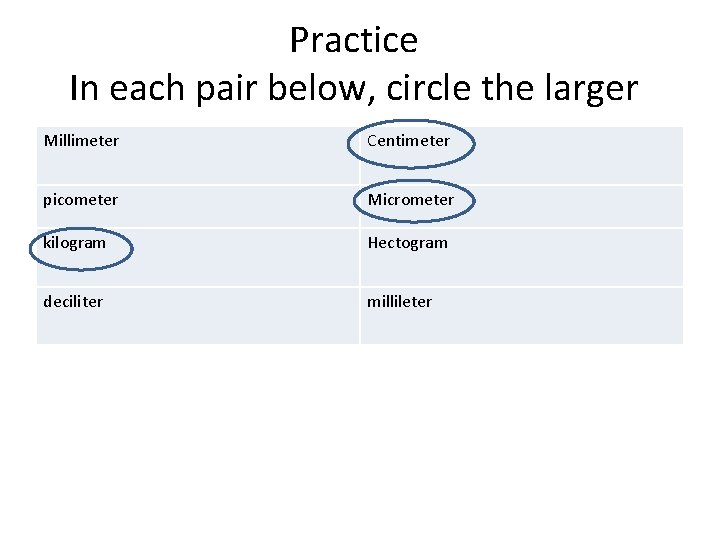
Practice In each pair below, circle the larger Millimeter Centimeter picometer Micrometer kilogram Hectogram deciliter millileter

Practice In each pair below, circle the larger Millimeter Centimeter picometer Micrometer kilogram Hectogram deciliter millileter

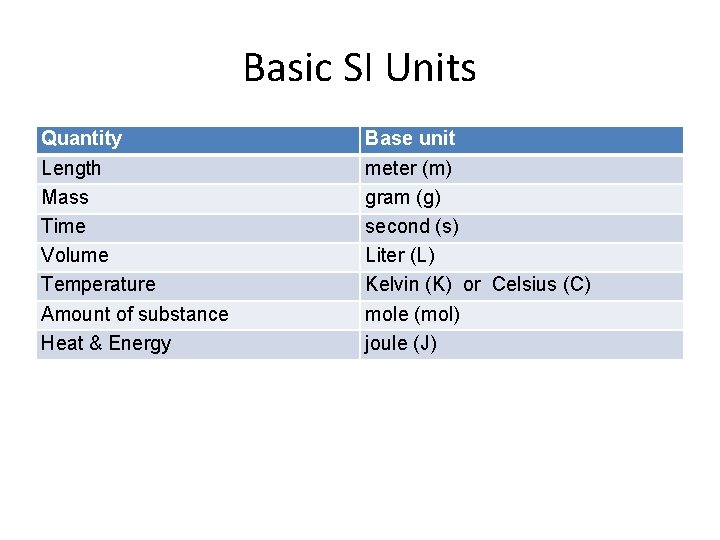
Basic SI Units Quantity Length Mass Time Volume Temperature Amount of substance Heat & Energy Base unit meter (m) gram (g) second (s) Liter (L) Kelvin (K) or Celsius (C) mole (mol) joule (J)

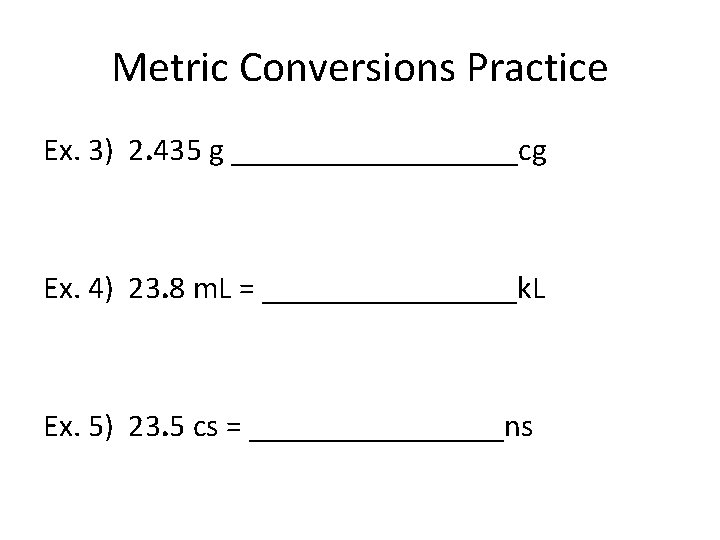
Metric Conversions Practice Ex. 3) 2. 435 g _________cg Ex. 4) 23. 8 m. L = ________k. L Ex. 5) 23. 5 cs = ________ns

Some Useful Conversions Length: 1 in = 2. 54 cm 1 mi = 5280 ft Volume: 1 cm 3 = 1 m. L 1 L = 1. 06 qt Weight: 1 kg = 2. 2 lb 16 oz = 1 lb 1 ton = 2000 lb


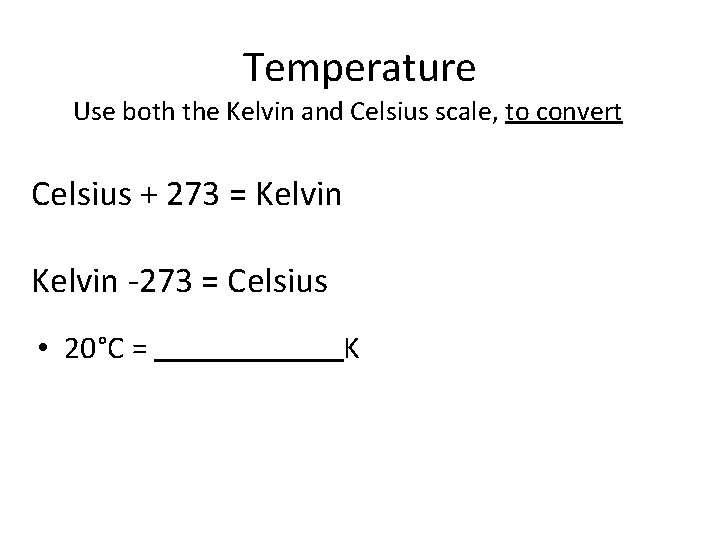
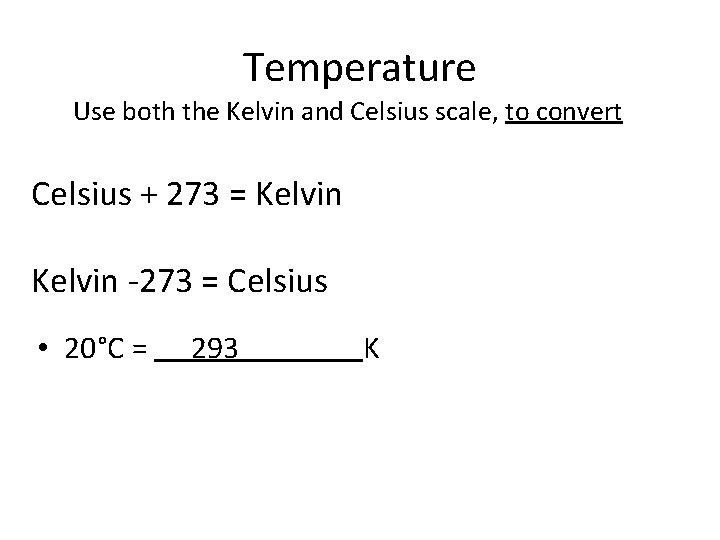
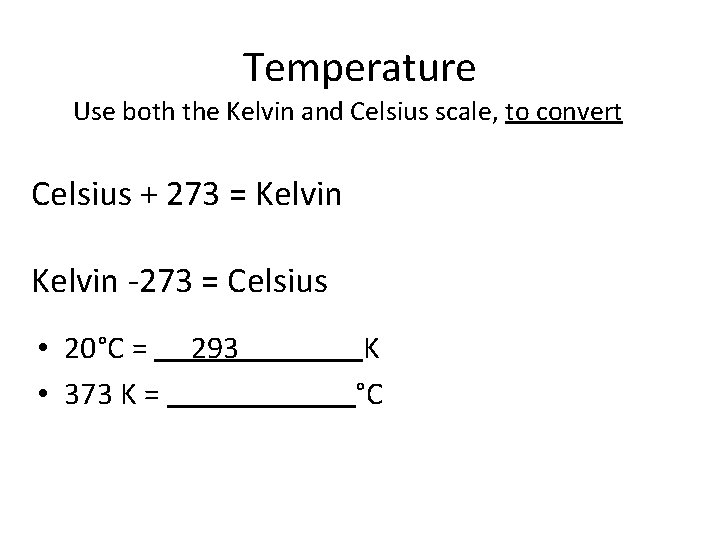
Temperature Use both the Kelvin and Celsius scale, to convert Celsius + 273 = Kelvin -273 = Celsius • 20°C = K

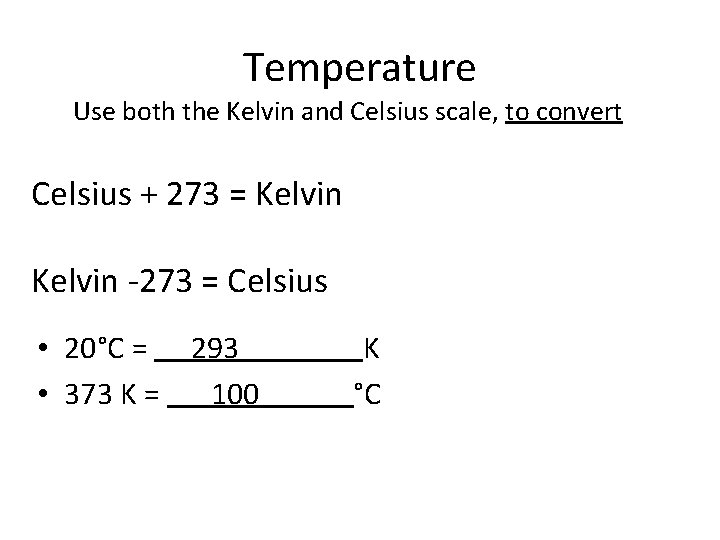
Temperature Use both the Kelvin and Celsius scale, to convert Celsius + 273 = Kelvin -273 = Celsius • 20°C = 293 K

Temperature Use both the Kelvin and Celsius scale, to convert Celsius + 273 = Kelvin -273 = Celsius • 20°C = 293 K • 373 K = °C

Temperature Use both the Kelvin and Celsius scale, to convert Celsius + 273 = Kelvin -273 = Celsius • 20°C = 293 K • 373 K = 100 °C

Volume: measured in cubic centimeters (cm ) or liters 3 • 1 liter (L) = 1 cubic decimeter (dm 3) = 1000 millileters (m. L) • 1 m. L= 1 cm 3

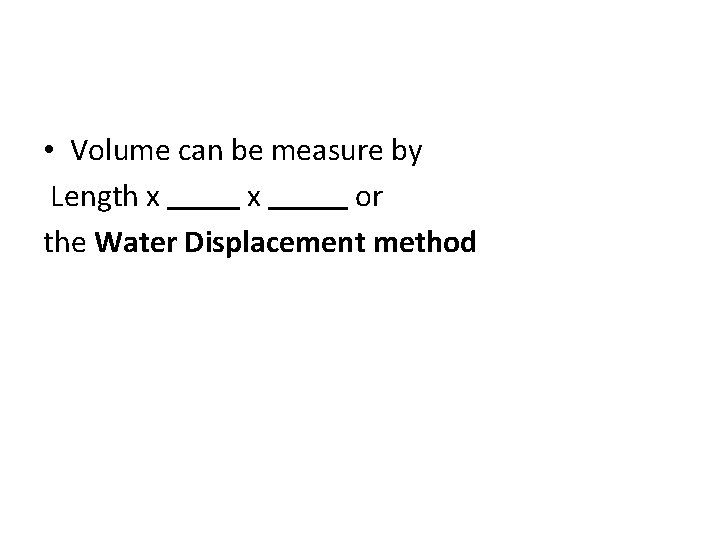
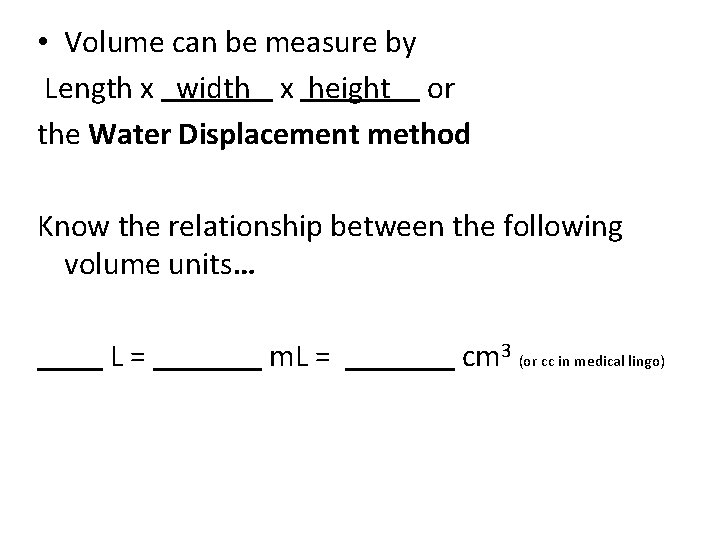
• Volume can be measure by Length x x or the Water Displacement method

• Volume can be measure by Length x width x or the Water Displacement method

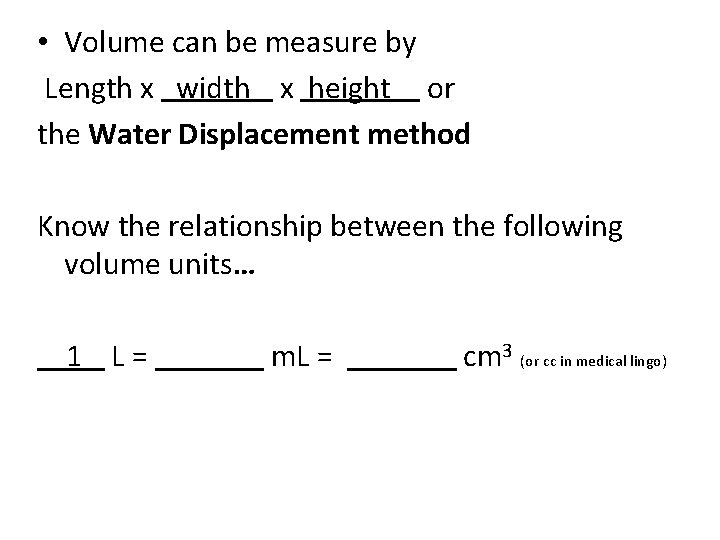
• Volume can be measure by Length x width x height or the Water Displacement method

• Volume can be measure by Length x width x height or the Water Displacement method Know the relationship between the following volume units… L = m. L = cm 3 (or cc in medical lingo)

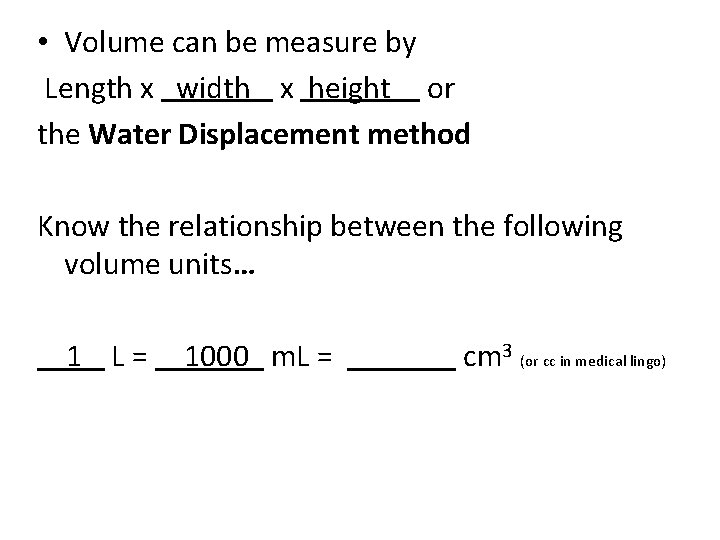
• Volume can be measure by Length x width x height or the Water Displacement method Know the relationship between the following volume units… 1 L = m. L = cm 3 (or cc in medical lingo)

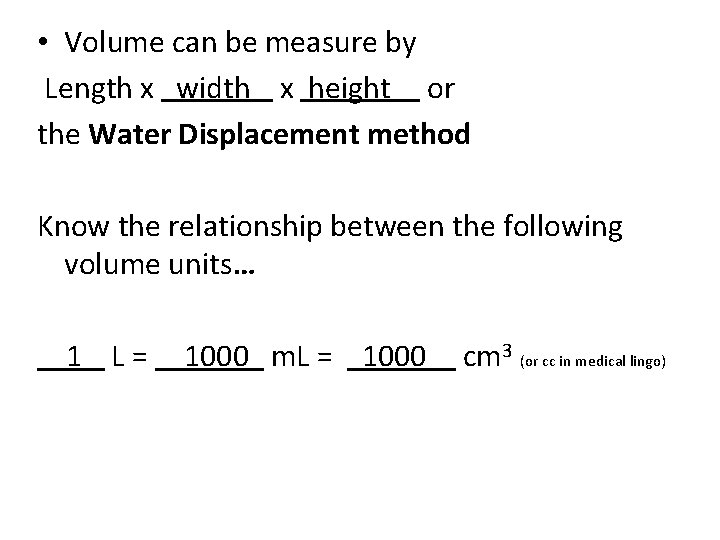
• Volume can be measure by Length x width x height or the Water Displacement method Know the relationship between the following volume units… 1 L = 1000 m. L = cm 3 (or cc in medical lingo)

• Volume can be measure by Length x width x height or the Water Displacement method Know the relationship between the following volume units… 1 L = 1000 m. L = 1000 cm 3 (or cc in medical lingo)

Density • Is the ratio of mass per unit of volume. How much matter is packed into a given amount of space • Density = mass/volume • D= m/v

• The Density of a substance stays regardless of the size of the sample. For example: if you cut a block of copper in half, you have decreased both the mass and volume, the ratio of the 2 stays the same. This is called an Intensive Physical Property.

• The Density of a substance stays constant regardless of the size of the sample. For example: if you cut a block of copper in half, you have decreased both the mass and volume, the ratio of the 2 stays the same. This is called an Intensive Physical Property.

• The appropriate units of density are: • for solids • for liquids

• The appropriate units of density are: • g/cm 3 for solids • for liquids

• The appropriate units of density are: • g/cm 3 for solids • g/m. L for liquids

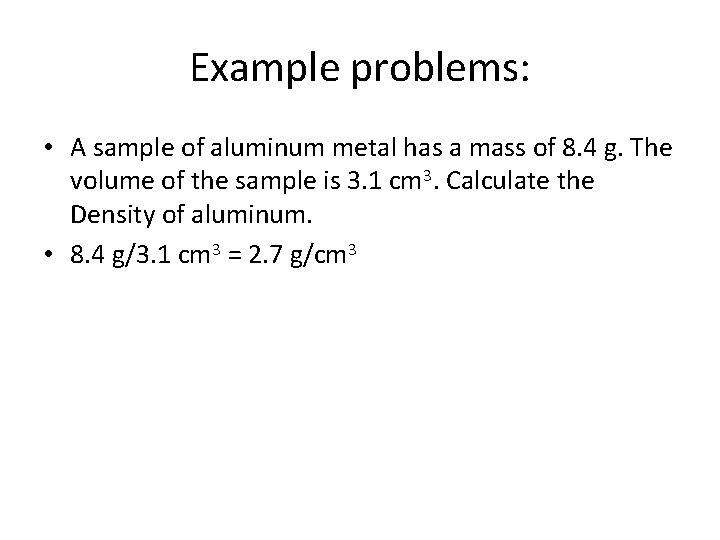
Example problems: • A sample of aluminum metal has a mass of 8. 4 g. The volume of the sample is 3. 1 cm 3. Calculate the Density of aluminum.

Example problems: • A sample of aluminum metal has a mass of 8. 4 g. The volume of the sample is 3. 1 cm 3. Calculate the Density of aluminum. • 8. 4 g/3. 1 cm 3 =

Example problems: • A sample of aluminum metal has a mass of 8. 4 g. The volume of the sample is 3. 1 cm 3. Calculate the Density of aluminum. • 8. 4 g/3. 1 cm 3 = 2. 7 g/cm 3

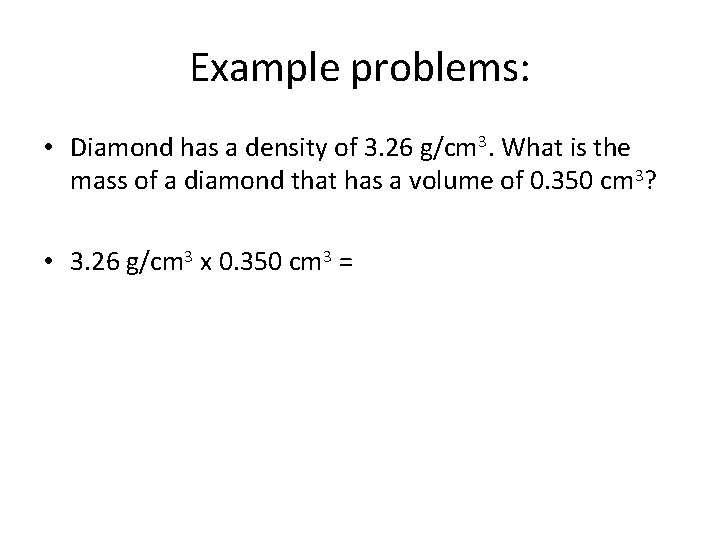
Example problems: • Diamond has a density of 3. 26 g/cm 3. What is the mass of a diamond that has a volume of 0. 350 cm 3?

Example problems: • Diamond has a density of 3. 26 g/cm 3. What is the mass of a diamond that has a volume of 0. 350 cm 3? • 3. 26 g/cm 3 x 0. 350 cm 3 =

Example problems: • Diamond has a density of 3. 26 g/cm 3. What is the mass of a diamond that has a volume of 0. 350 cm 3? • 3. 26 g/cm 3 x 0. 350 cm 3 = 1. 14 g

Example problems: • What is the volume of a sample of liquid mercury that has a mass of 76. 2 g, given that the density of mercury is 13. 6 g/m. L?

Example problems: • What is the volume of a sample of liquid mercury that has a mass of 76. 2 g, given that the density of mercury is 13. 6 g/m. L? 76. 2 g = 13. 6 g/m. L

Example problems: • What is the volume of a sample of liquid mercury that has a mass of 76. 2 g, given that the density of mercury is 13. 6 g/m. L? 76. 2 g = 5. 60 m. L 13. 6 g/m. L

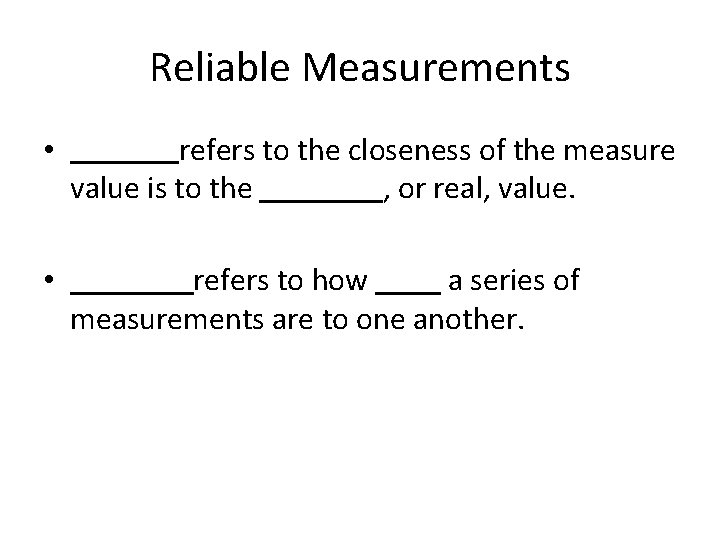
Reliable Measurements • refers to the closeness of the measure value is to the , or real, value. • refers to how a series of measurements are to one another.

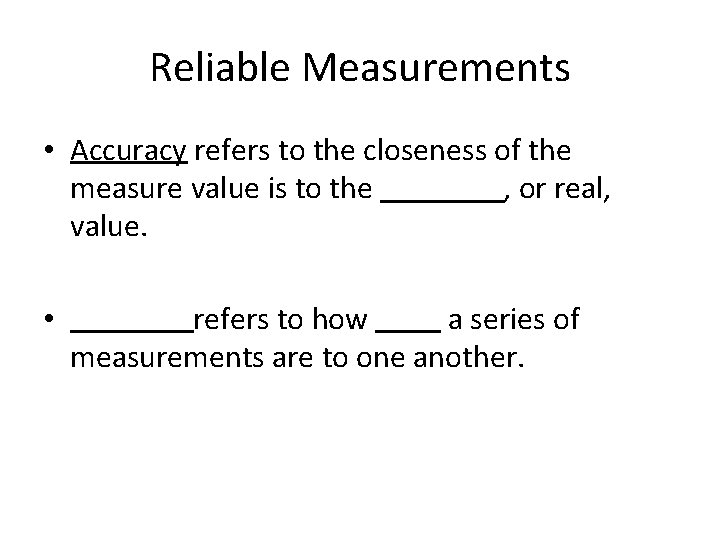
Reliable Measurements • Accuracy refers to the closeness of the measure value is to the , or real, value. • refers to how a series of measurements are to one another.

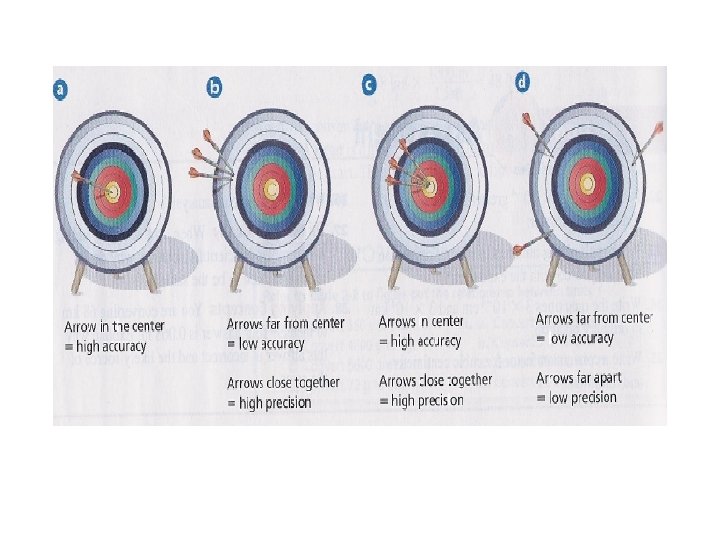
Reliable Measurements • Accuracy refers to the closeness of the measure value is to the accepted, or real, value. • refers to how a series of measurements are to one another.

Reliable Measurements • Accuracy refers to the closeness of the measure value is to the accepted, or real, value. • Precision refers to how a series of measurements are to one another.

Reliable Measurements • Accuracy refers to the closeness of the measure value is to the accepted, or real, value. • Precision refers to how close a series of measurements are to one another.


• is calculated by subtracting the value from the value.

• Error is calculated by subtracting the experimental value from the accepted value.

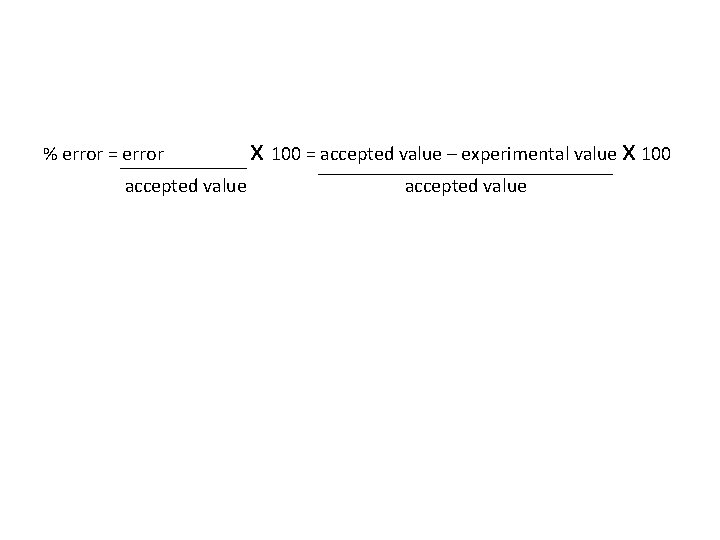
• The is the ratio of an error to an accepted value.

• The percent error is the ratio of an error to an accepted value.

% error = error x 100 = accepted value – experimental value x 100 accepted value

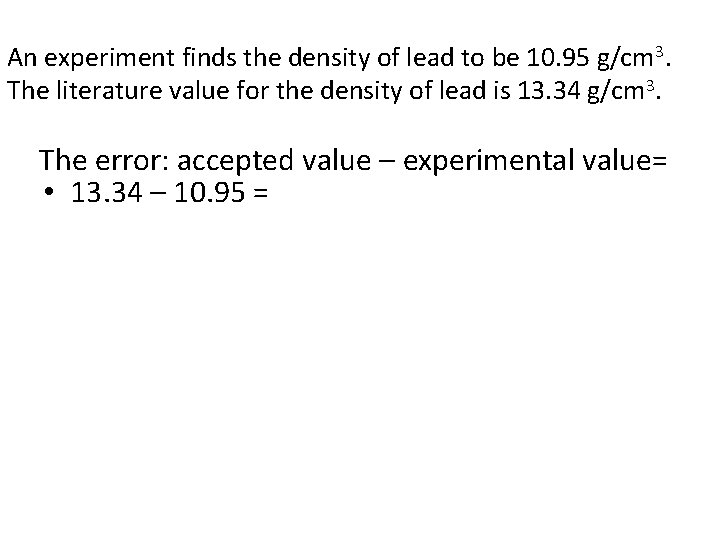
Example • An experiment finds the density of lead to be 10. 95 g/cm 3. The literature value for the density of lead is 13. 34 g/cm 3.

An experiment finds the density of lead to be 10. 95 g/cm 3. The literature value for the density of lead is 13. 34 g/cm 3. The error: accepted value – experimental value= • 13. 34 – 10. 95 =

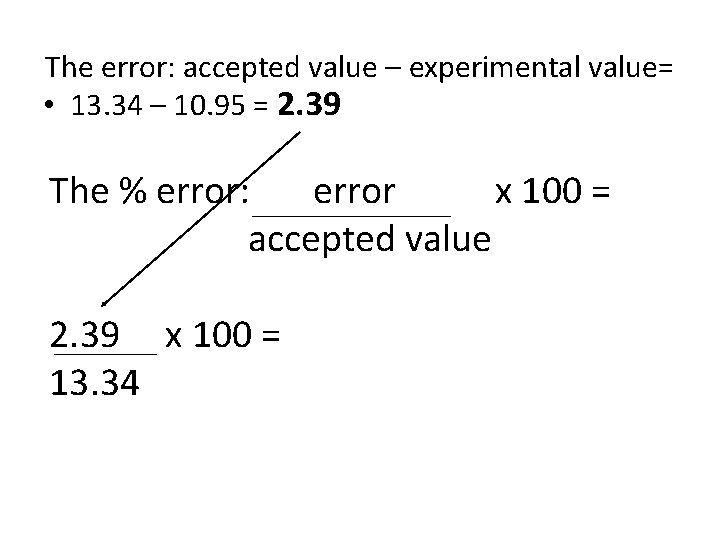
An experiment finds the density of lead to be 10. 95 g/cm 3. The literature value for the density of lead is 13. 34 g/cm 3. The error: accepted value – experimental value= • 13. 34 – 10. 95 = 2. 39

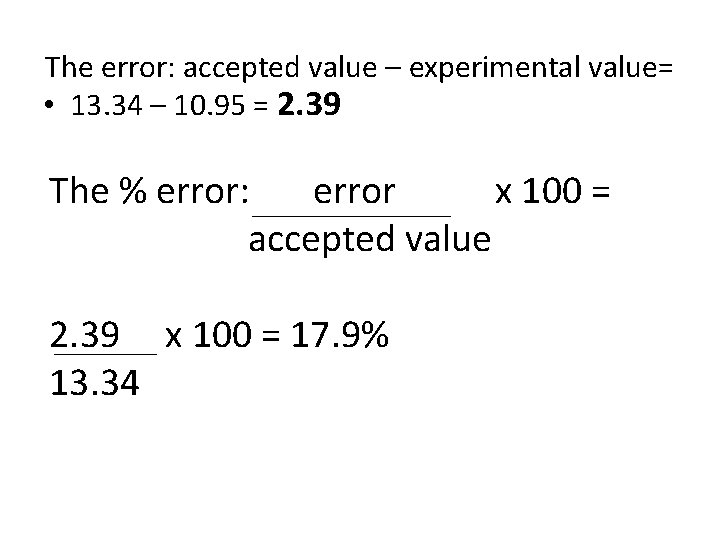
The error: accepted value – experimental value= • 13. 34 – 10. 95 = 2. 39 The % error: error x 100 = accepted value 2. 39 x 100 = 13. 34

The error: accepted value – experimental value= • 13. 34 – 10. 95 = 2. 39 The % error: error x 100 = accepted value 2. 39 x 100 = 17. 9% 13. 34

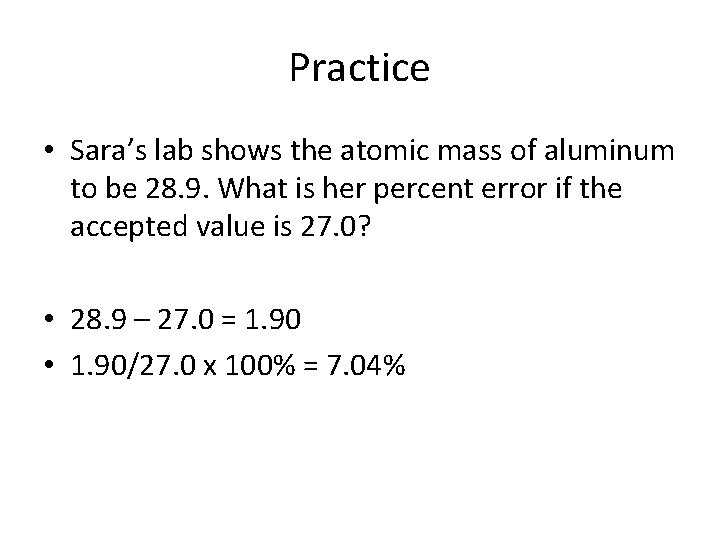
Practice • Sara’s lab shows the atomic mass of aluminum to be 28. 9. What is her percent error if the accepted value is 27. 0?

Practice • Sara’s lab shows the atomic mass of aluminum to be 28. 9. What is her percent error if the accepted value is 27. 0? • 28. 9 – 27. 0 =

Practice • Sara’s lab shows the atomic mass of aluminum to be 28. 9. What is her percent error if the accepted value is 27. 0? • 28. 9 – 27. 0 = 1. 90

Practice • Sara’s lab shows the atomic mass of aluminum to be 28. 9. What is her percent error if the accepted value is 27. 0? • 28. 9 – 27. 0 = 1. 90 • 1. 90/27. 0 x 100% =

Practice • Sara’s lab shows the atomic mass of aluminum to be 28. 9. What is her percent error if the accepted value is 27. 0? • 28. 9 – 27. 0 = 1. 90 • 1. 90/27. 0 x 100% = 7. 04%

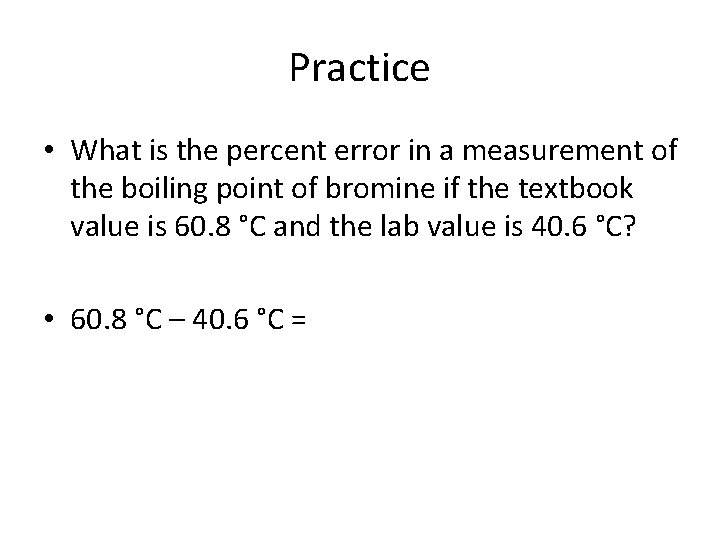
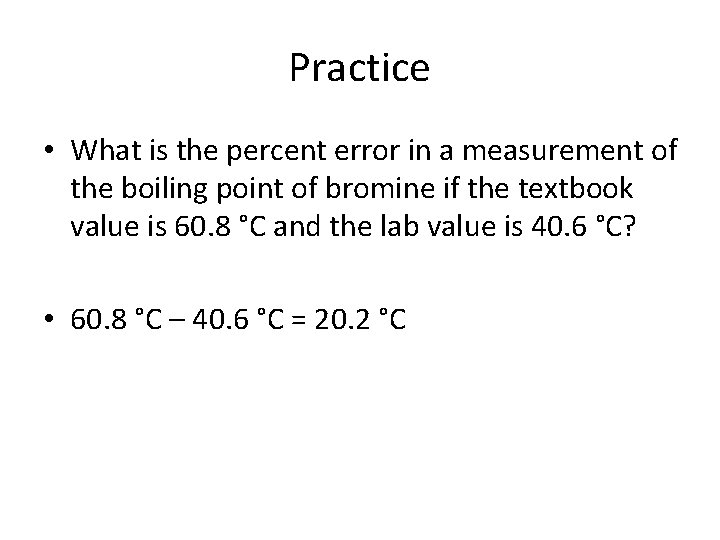
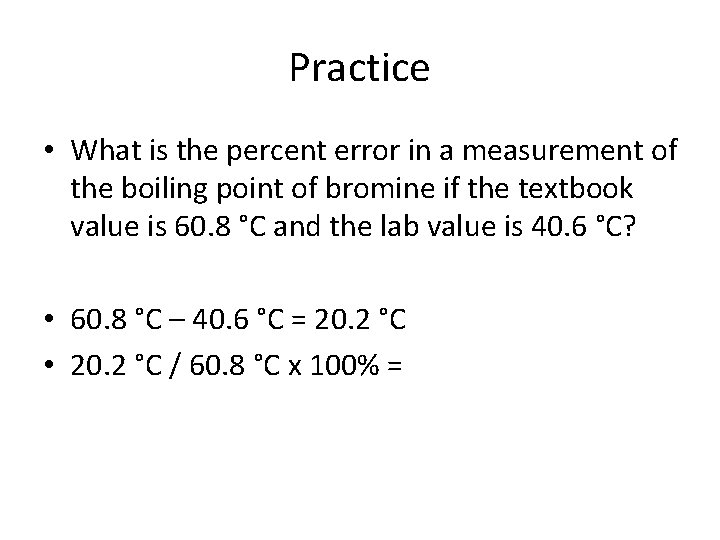
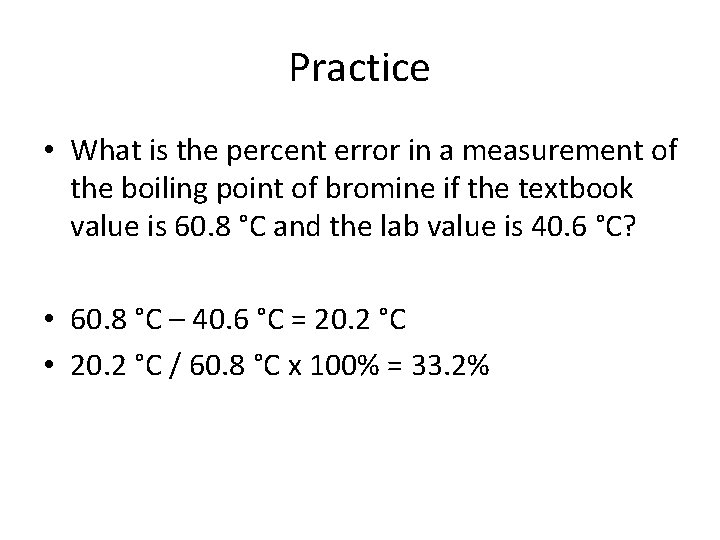
Practice • What is the percent error in a measurement of the boiling point of bromine if the textbook value is 60. 8 °C and the lab value is 40. 6 °C?

Practice • What is the percent error in a measurement of the boiling point of bromine if the textbook value is 60. 8 °C and the lab value is 40. 6 °C? • 60. 8 °C – 40. 6 °C =

Practice • What is the percent error in a measurement of the boiling point of bromine if the textbook value is 60. 8 °C and the lab value is 40. 6 °C? • 60. 8 °C – 40. 6 °C = 20. 2 °C

Practice • What is the percent error in a measurement of the boiling point of bromine if the textbook value is 60. 8 °C and the lab value is 40. 6 °C? • 60. 8 °C – 40. 6 °C = 20. 2 °C • 20. 2 °C / 60. 8 °C x 100% =

Practice • What is the percent error in a measurement of the boiling point of bromine if the textbook value is 60. 8 °C and the lab value is 40. 6 °C? • 60. 8 °C – 40. 6 °C = 20. 2 °C • 20. 2 °C / 60. 8 °C x 100% = 33. 2%