Diffusion and Osmosis Dr Shakha Sharda Diffusion The






- Slides: 17

Diffusion and Osmosis Dr. Shakha Sharda

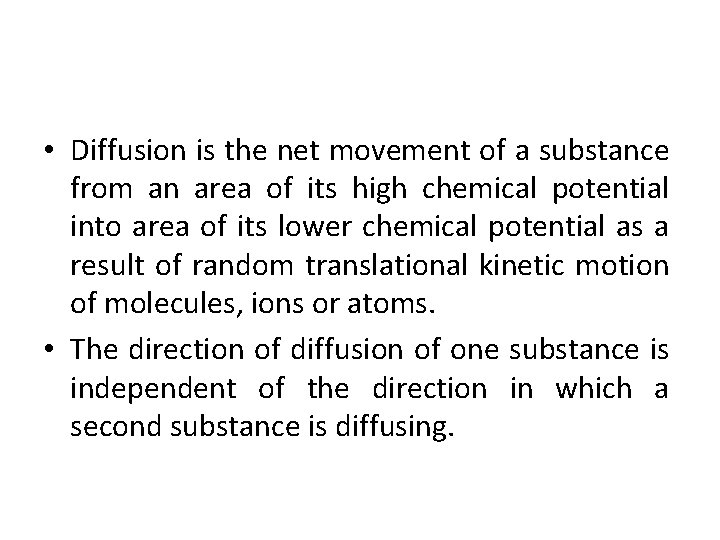
Diffusion • The movement of molecules or ions of a solute or a solvent (solid, liquid or gas from the region of its higher concentration to that of its lower concentration. • Molecules or ions are (high pressure when concentrated) diffused out from region of high partial pressure to low partial pressure due to their inherent kinetic energy and move till equilibrium is obtained.

• Diffusion is the net movement of a substance from an area of its high chemical potential into area of its lower chemical potential as a result of random translational kinetic motion of molecules, ions or atoms. • The direction of diffusion of one substance is independent of the direction in which a second substance is diffusing.

Diffusion Pressure • Potential ability of a molecule or ion (solid, liquid or gas) to diffuse from an area of its greatest concentration to an area of lesser concentration. • The diffusion pressure is directly proportional to the concentration or the number of diffusing particles. • Greater the concentration of diffusing particles, greater is their diffusion pressure.

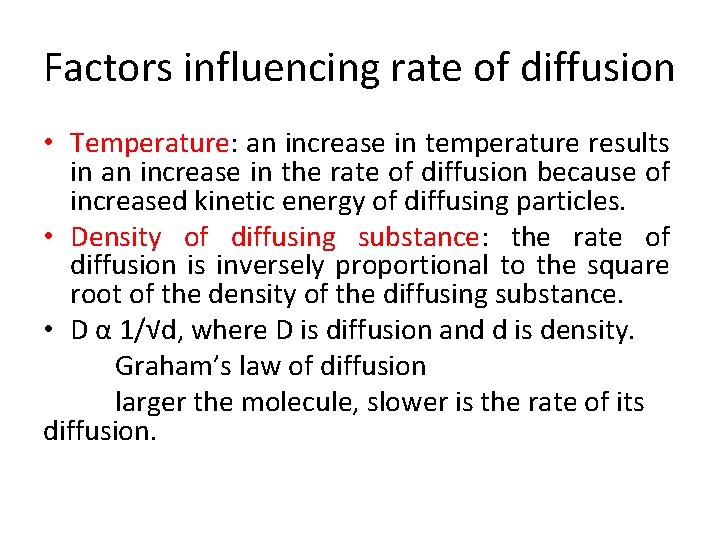
Factors influencing rate of diffusion • Temperature: an increase in temperature results in an increase in the rate of diffusion because of increased kinetic energy of diffusing particles. • Density of diffusing substance: the rate of diffusion is inversely proportional to the square root of the density of the diffusing substance. • D α 1/√d, where D is diffusion and d is density. Graham’s law of diffusion larger the molecule, slower is the rate of its diffusion.

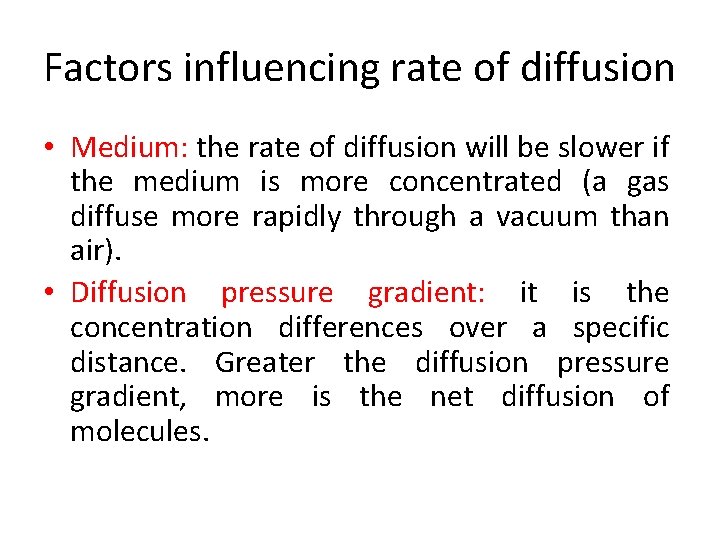
Factors influencing rate of diffusion • Medium: the rate of diffusion will be slower if the medium is more concentrated (a gas diffuse more rapidly through a vacuum than air). • Diffusion pressure gradient: it is the concentration differences over a specific distance. Greater the diffusion pressure gradient, more is the net diffusion of molecules.

Significance of diffusion in plants • The exchange of gases, carbon dioxide intake and oxygen output in photosynthesis and carbon dioxide output and oxygen intake in photosynthesis, takes place through diffusion. • Helps in the transpiration of water vapours. • Ions are absorbed by the simple diffusion during passive salt uptake. • An effective means of transport of substances over a short distance and helps in the translocation of food materials.

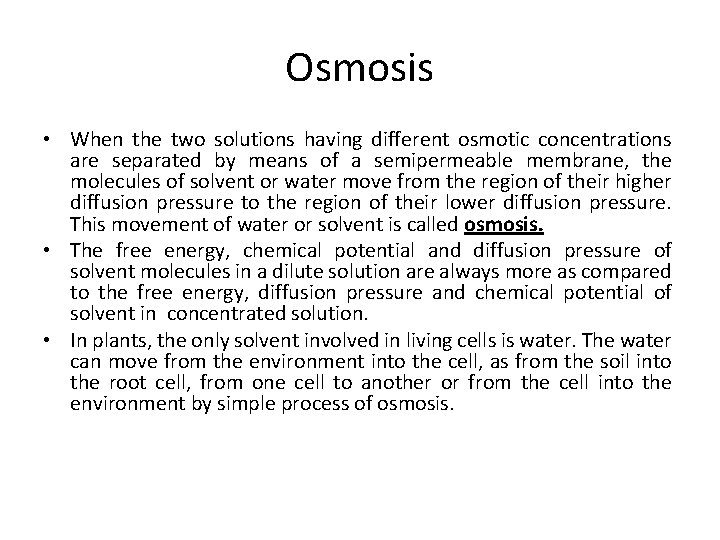
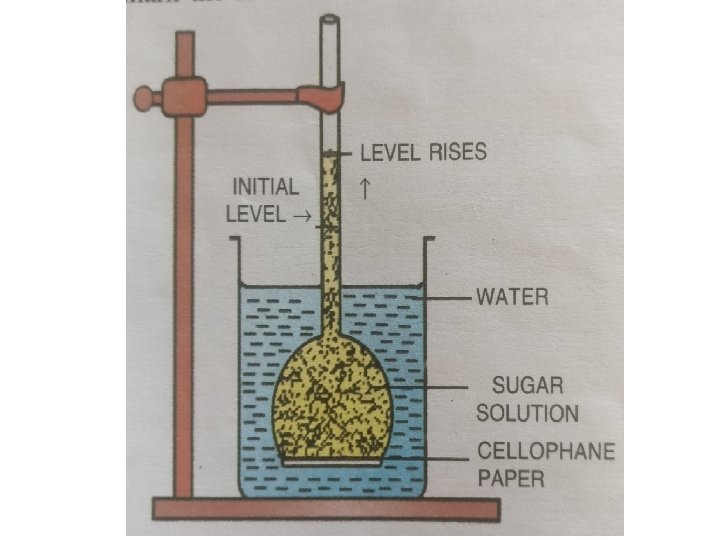
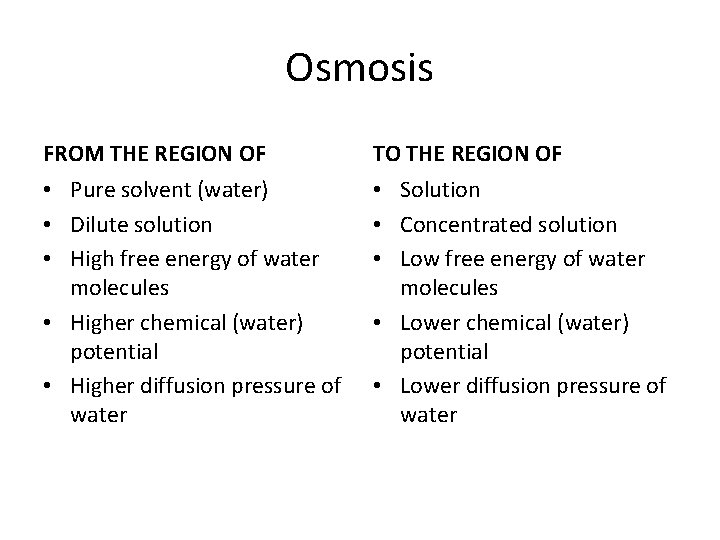
Osmosis • When the two solutions having different osmotic concentrations are separated by means of a semipermeable membrane, the molecules of solvent or water move from the region of their higher diffusion pressure to the region of their lower diffusion pressure. This movement of water or solvent is called osmosis. • The free energy, chemical potential and diffusion pressure of solvent molecules in a dilute solution are always more as compared to the free energy, diffusion pressure and chemical potential of solvent in concentrated solution. • In plants, the only solvent involved in living cells is water. The water can move from the environment into the cell, as from the soil into the root cell, from one cell to another or from the cell into the environment by simple process of osmosis.


Osmosis FROM THE REGION OF TO THE REGION OF • Pure solvent (water) • Dilute solution • High free energy of water molecules • Higher chemical (water) potential • Higher diffusion pressure of water • Solution • Concentrated solution • Low free energy of water molecules • Lower chemical (water) potential • Lower diffusion pressure of water

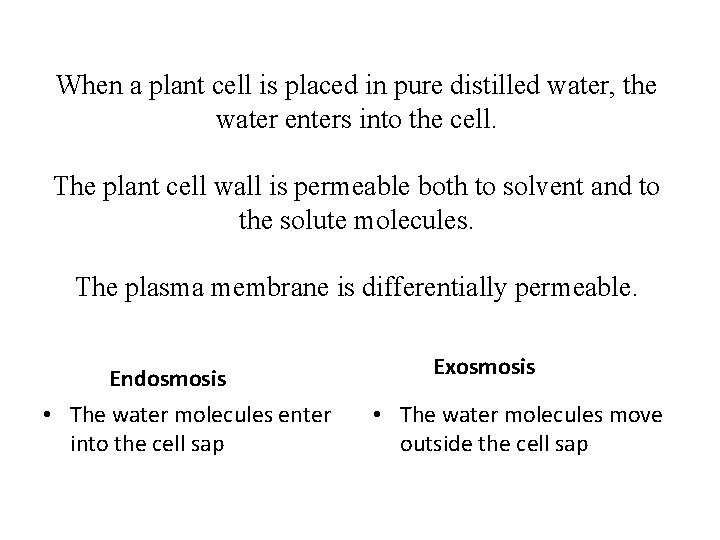
When a plant cell is placed in pure distilled water, the water enters into the cell. The plant cell wall is permeable both to solvent and to the solute molecules. The plasma membrane is differentially permeable. Endosmosis • The water molecules enter into the cell sap Exosmosis • The water molecules move outside the cell sap

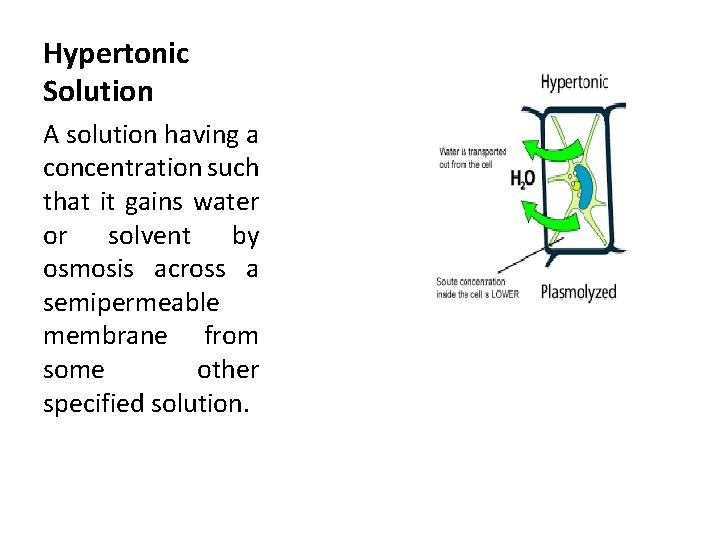
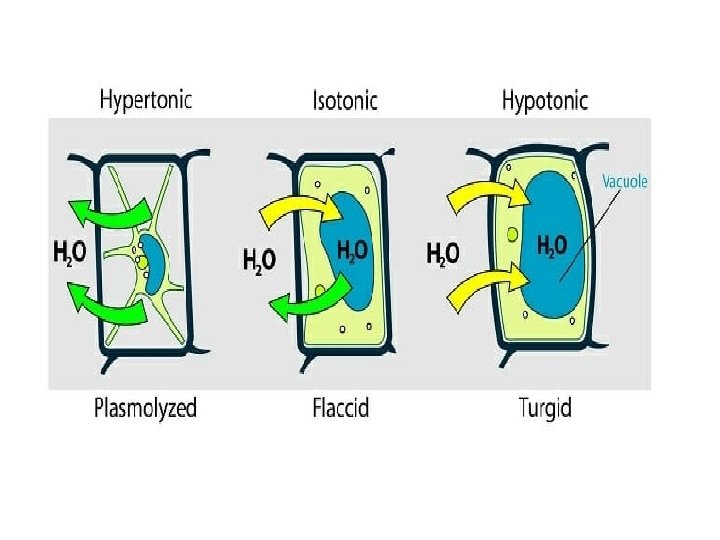
Hypertonic Solution A solution having a concentration such that it gains water or solvent by osmosis across a semipermeable membrane from some other specified solution.

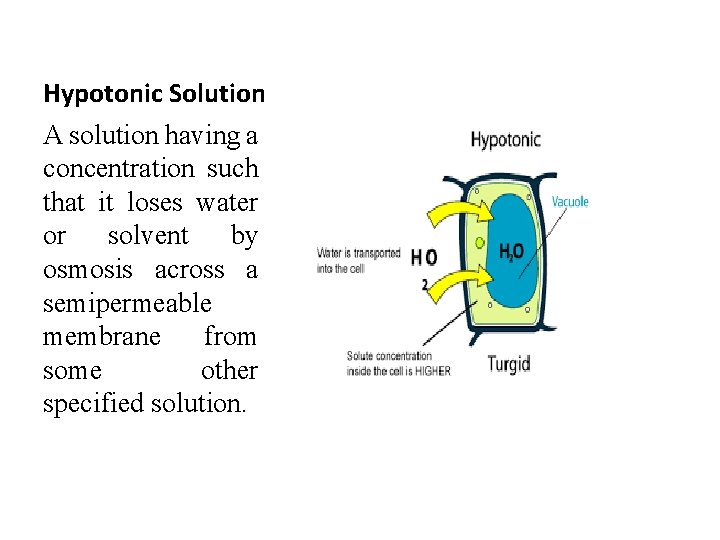
Hypotonic Solution A solution having a concentration such that it loses water or solvent by osmosis across a semipermeable membrane from some other specified solution.

Isotonic Solution A solution having a concentration such that it neither gains nor loses water or solvent by osmosis across a semipermeable membrane from some other specified solution.


Osmotic Pressure • Osmotic pressure is the pressure exerted upon the solution to prevent the flow of solvent into it to across a semipermeable membrane. • Maximum amount of pressure that can be developed in a solution separated from pure water by a semipermeable membrane is called an osmotic pressure. • It is usually measured in pascals, Pa (1 Pa = 1 Newton/m 2 Osmotic pressure=CST(C=molar concentration of solution, S is solution constant; 0. 082 and T is absolute temperature; +t 0 C+273 only in case of non -electrolytes e. g. sucrose.

Importance of Osmosis in plants • Osmosis is important in the absorption of water by plants. • Osmosis help in cell to cell movement of water throughout the plant body. • The rigidity of plant organs (shape and form of organism) is maintained through osmosis. • Turgidity and expansion of leaves occur due to osmotic pressure. • Growing points of root remain turgid because of osmosis and are thus, able to penetrate the soil particles. • The resistance of plants to drought and frost is due to osmotic pressure of their cells. • Movement of plant and plant parts, bursting of many fruits and sporangia occur due to osmosis. • Opening and closing of stomata is affected due to osmosis.