DICOM Standards Committee Report on Japan Activities Hiroshi







- Slides: 13

DICOM Standards Committee Report on Japan Activities Hiroshi Sakamoto / JSRT Megumi Kondo / JAHIS Akihiro Yomoda/ JIRA 2016 -DEC-01 DSC@ RSNA Chicago 2016/12/01 DICOM DSC

Contents (activities since last report (2016/SEP)) 1. JIRA - DICOM committee 1. Seminars 2. Healthcare software 2. JSRT - Medical Informatics Section 1. Autumn Congress 2. Seminars 3. JAHIS - Informatics System Section 1. Pathology and Endoscope 4. IHE – Japan 1. Connectathon 2016 and 2017 2016/12/01 DICOM DSC 2

1. JIRA DICOM committee 1. Seminars – DICOM book Seminar • Dec. 17 • Feb. 04 • target : beginners, RT and Vendors – New Tech Seminar • Aug. 30 • Theme : Application of AI to medical field – Government : Healthcare of next era – University : Deep learning – Vendor : Dr. Watson 2016/12/01 DICOM DSC 3

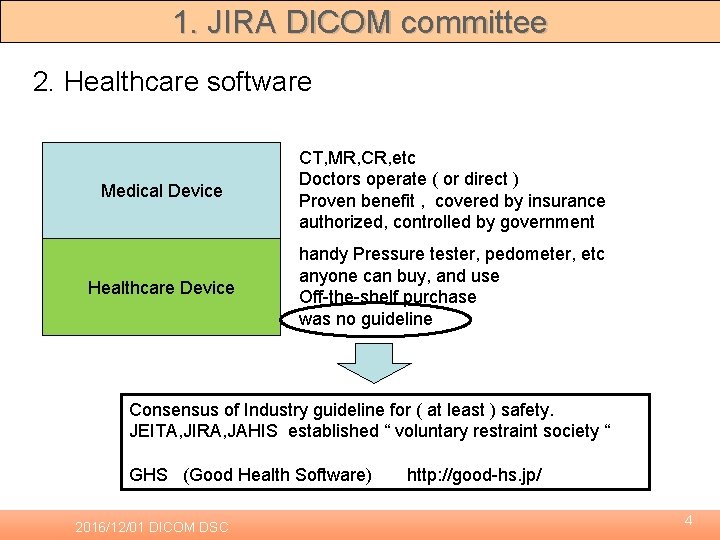
1. JIRA DICOM committee 2. Healthcare software Medical Device CT, MR, CR, etc Doctors operate ( or direct ) Proven benefit , covered by insurance authorized, controlled by government Healthcare Device handy Pressure tester, pedometer, etc anyone can buy, and use Off-the-shelf purchase was no guideline Consensus of Industry guideline for ( at least ) safety. JEITA, JIRA, JAHIS established “ voluntary restraint society “ GHS (Good Health Software) 2016/12/01 DICOM DSC http: //good-hs. jp/ 4

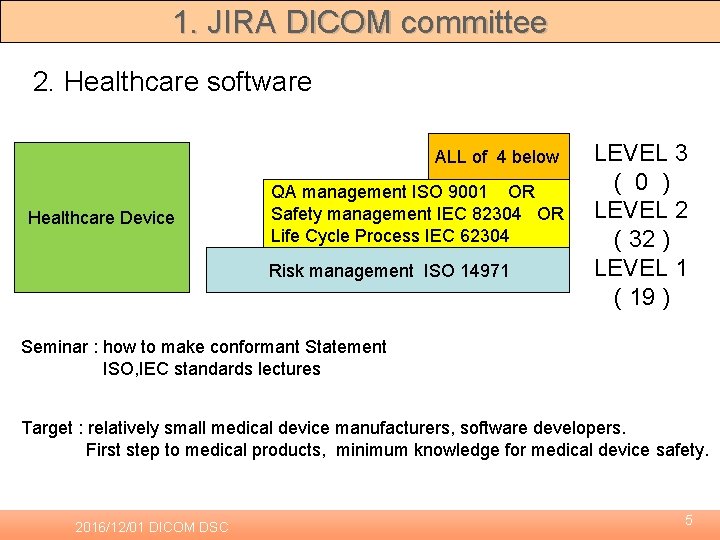
1. JIRA DICOM committee 2. Healthcare software ALL of 4 below Healthcare Device QA management ISO 9001 OR Safety management IEC 82304 OR Life Cycle Process IEC 62304 Risk management ISO 14971 LEVEL 3 ( 0 ) LEVEL 2 ( 32 ) LEVEL 1 ( 19 ) Seminar : how to make conformant Statement ISO, IEC standards lectures Target : relatively small medical device manufacturers, software developers. First step to medical products, minimum knowledge for medical device safety. 2016/12/01 DICOM DSC 5

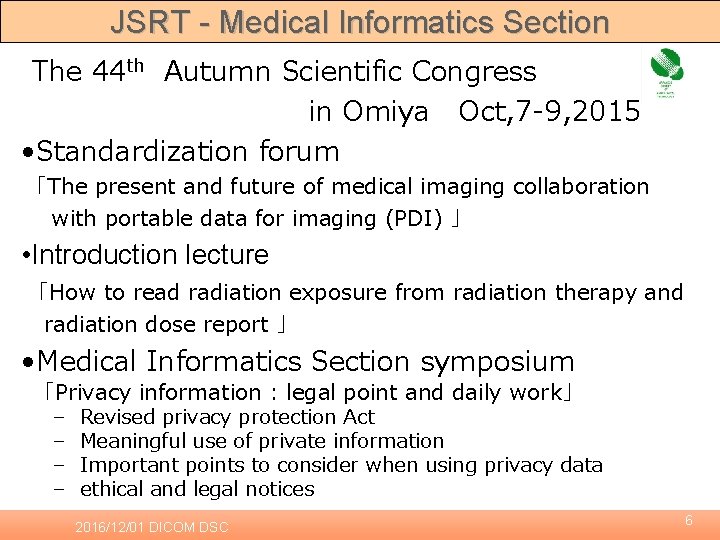
JSRT - Medical Informatics Section The 44 th Autumn Scientific Congress in Omiya Oct, 7 -9, 2015 • Standardization forum 「The present and future of medical imaging collaboration with portable data for imaging (PDI) 」 • Introduction lecture 「How to read radiation exposure from radiation therapy and radiation dose report 」 • Medical Informatics Section symposium 「Privacy information : legal point and daily work」 – – Revised privacy protection Act Meaningful use of private information Important points to consider when using privacy data ethical and legal notices 2016/12/01 DICOM DSC 6

JSRT - Medical Informatics Section Recent Activities of JSRT Medical Informatics Section • PACS Basic seminar for beginners about DICOM, RIS, PACS, DICOM, Informatics, Network, Guidelines 5 th 6 th Jul. 9, 2016 Sep. 3, 2016 in Yokohama in Kagoshima & Okinawa(satellite) • PACS Specialist seminar for seniors about DICOM, JJ 1017 , DICOM Update , BCP 17 th Jun. 18, 2016 in Sapporo 18 th Sep. 24, 2016 in Kanazawa 19 th Nov. 10, 2016 in Hiroshima 2016/12/01 DICOM DSC 7

JAHIS - Informatics System Section • Pathology domain – Introduce DICOM for WSI in the Annual Meeting of the Japanese Society of Digital Pathology on September 10 – Attend WG-26 F 2 F Meeting in San Diego on October 23 • Endoscopy domain – Review the Supplement 195 which introduces new HEVC Transfer Syntaxes – Attend WG-13 t-cons about NWIP of Real-Time Video 2016/12/01 DICOM DSC 8

IHE – Japan Connectathon 2016 • Connectathon 2016 – 2016/SEP/13 -19 – fee member Asakusa, Tokyo non-member US$4, 000+1, 000/sys* US$5, 000+2, 000/sys ( * : n systems free when joined n times in past ) – 43 companies 2016/12/01 DICOM DSC 9

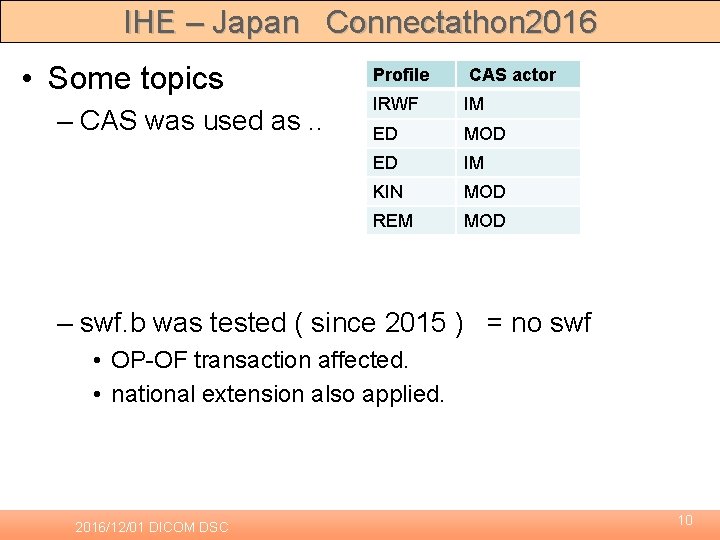
IHE – Japan Connectathon 2016 • Some topics – CAS was used as. . Profile CAS actor IRWF IM ED MOD ED IM KIN MOD REM MOD – swf. b was tested ( since 2015 ) = no swf • OP-OF transaction affected. • national extension also applied. 2016/12/01 DICOM DSC 10

IHE – Japan Connectathon 2017 (PLAN) Connectathon 2017 (PLAN) • 2017/SEP/25 -29 (MON-FRI) – Location : Asakusa, Downtown Tokyo – Open House day SEP-27 (WED) 2016/12/01 DICOM DSC 11

DSC Asia 2017/SEP/ 25(MON) 26(TUE) 27(WED)+ candidate WG-10, 29 and welcome party DSC WG’s <= JP-CAT lecture Location: JIRA office ( Iidabashi, Tokyo) and/or JAHIS office ( Shimbashi, Tokyo) 2016/12/01 DICOM DSC 12

Report on Japan Activities JSRT : Japan Society of Radiological Technology JAHIS : Japanese Association of Healthcare Information Systems Industry JIRA : Japan Medical Imaging and Radiological Systems Industries Association 2016/12/01 DICOM DSC 13
 Dicomeye
Dicomeye Silvio hiroshi nakao
Silvio hiroshi nakao Game theory ffxiv
Game theory ffxiv Hiroshi kazato
Hiroshi kazato Dicom structured report
Dicom structured report Dicom xml
Dicom xml Dicom structured report
Dicom structured report Dicom2
Dicom2 House committee on unamerican activities
House committee on unamerican activities Taft-hartley act
Taft-hartley act House committee on unamerican activities
House committee on unamerican activities German accounting standards
German accounting standards International accounting standards committee
International accounting standards committee The name of yashpal committee report (1993 is)
The name of yashpal committee report (1993 is)