Understanding the DICOM SR Supplement DICOM Structured Reporting
























- Slides: 75

Understanding the DICOM SR Supplement DICOM Structured Reporting Workshop March 29 -30, 2000 Donald E. Van Syckle _____________ DICOM WG 6 Chairman Director, Merge. Link Professional Services m 1

DICOM Structured Reporting (SR) u How About DICOM Reporting? u Results Management – Get Prior Reports – DICOM Since ‘ 93 But Not Widely Implemented Structured Reporting – Standardized Early 2000 (April) – Simple Reporting » Basic and Enchanced – Complex Reporting » Comprehensive Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 2 m

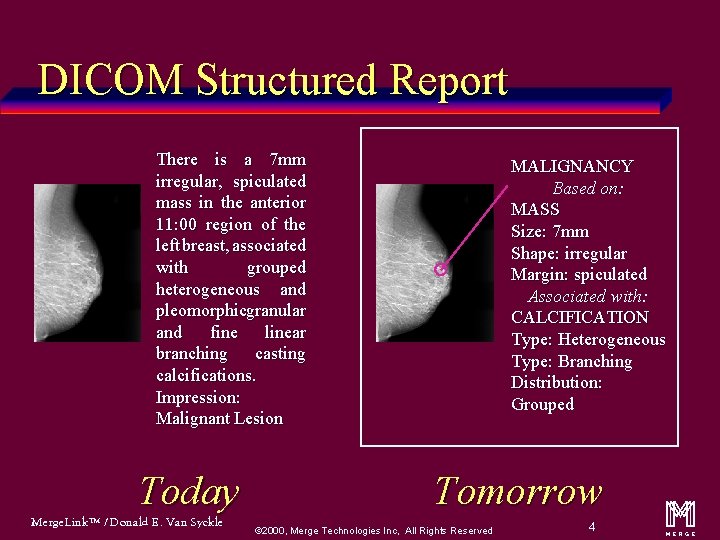
Today’s Reporting There is a 7 mm irregular, spiculated mass in the anterior 11: 00 region of the left breast, associated with grouped heterogeneous and pleomorphic granular and fine linear branching casting calcifications. Impression: Malignant Lesion Free form text only Not linked with images or other clinical data Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 3 m

DICOM Structured Report There is a 7 mm irregular, spiculated mass in the anterior 11: 00 region of the left breast, associated with grouped heterogeneous and pleomorphicgranular and fine linear branching casting calcifications. Impression: Malignant Lesion Today Merge. Link™ / Donald E. Van Syckle MALIGNANCY Based on: MASS Size: 7 mm Shape: irregular Margin: spiculated Associated with: CALCIFICATION Type: Heterogeneous Type: Branching Distribution: Grouped Tomorrow ã 2000, Merge Technologies Inc, All Rights Reserved 4 m

What is Structured Report? u A “Databaseable Document” which: – Provide unambiguous “semantic” documentation of diagnosis – Provide context such as: (scheduled procedure, observer, previous reports and images…) – Link text with images, waveforms, audio, measurements – Coded entry using standardized or private lexicons – Flexible enough to go beyond radiology imaging A“Databaseable Document” facilities computer outcome analysis Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 5 m

What Isn’t DICOM SR? MALIGNANCY Based on: MASS Size: 7 mm Shape: irregular Margin: spiculated Associated with: CALCIFICATION Type: Heterogeneous Type: Branching Distribution: Grouped …it isn’t a document presentation standard! Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 6 m

Key Concepts and Terms to Learn! u SR IOD Query/Retrieve Model Coded Entries u Content Items u Value Types u Relationship Types u Observation Context u u Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 7 m

Not Done Yet! Key Concepts and Terms to Learn u u u Merge. Link™ / Donald E. Van Syckle SR Document General SR Document Content Modification Rules Basic Structured Reporting Enhanced Structured Reporting Comprehensive Structured Reporting ã 2000, Merge Technologies Inc, All Rights Reserved 8 m

Does DICOM SR IOD follow the same model as an image? u. Yes! Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 9 m

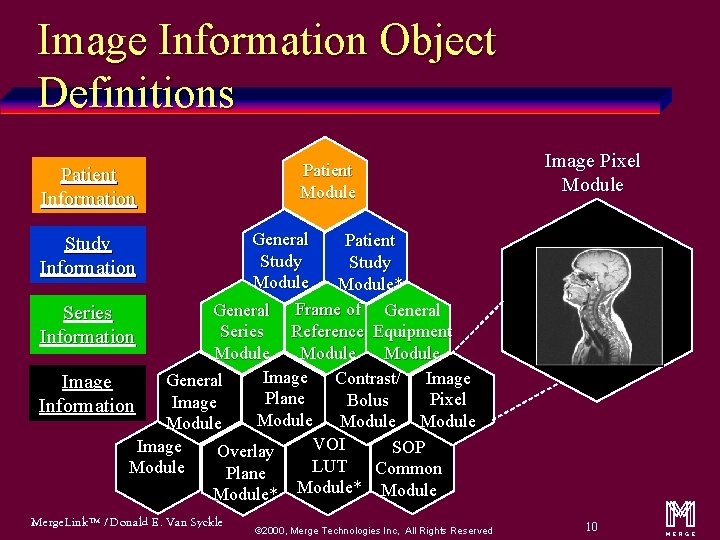
Image Information Object Definitions Patient Information Patient Module Image Pixel Module General Patient Study Module* General Frame of General Series Reference Equipment Series Information Module Image Contrast/ Image General Image Plane Pixel Bolus Image Information Module VOI Image SOP Overlay LUT Module Common Plane Module* Module Study Information Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 10 m

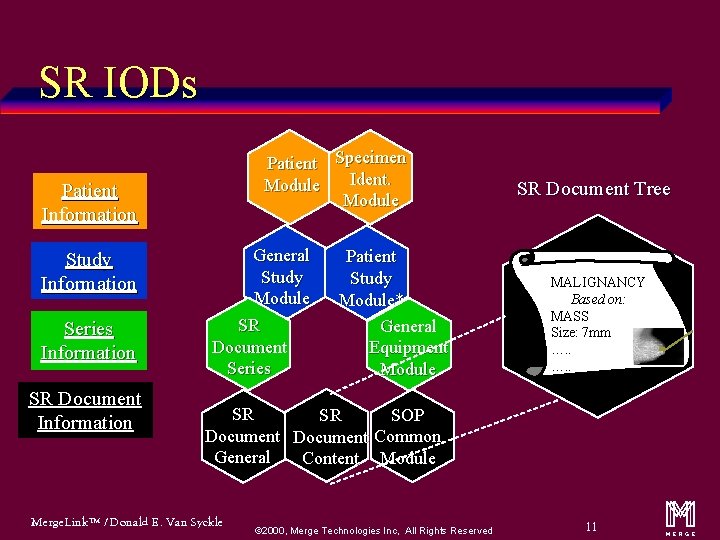
SR IODs Patient Specimen Ident. Module Patient Information Study Information Series Information SR Document Information General Study Module SR Document Series Patient Study Module* General Equipment Module SR Document Tree MALIGNANCY Based on: MASS Size: 7 mm …. . SR SOP SR Document Common General Content Module Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 11 m

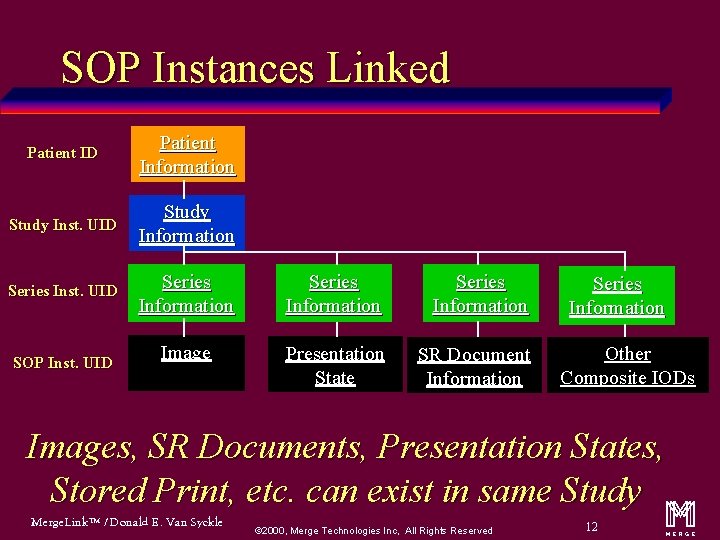
SOP Instances Linked Patient ID Patient Information Study Inst. UID Study Information Series Inst. UID Series Information Image Presentation State SR Document Information SOP Inst. UID Series Information Other Composite IODs Images, SR Documents, Presentation States, Stored Print, etc. can exist in same Study Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 12 m

SR IOD and Query/Retrieve Model u SR IODs mirror the Composite IOD model (i. e. images). u SR objects are able to be linked to images, waveforms, stored print, presentations state, RT plans… u “Pushing” SR SOP Instances accomplished via C-STORE u “Pulling” SR SOP Instances accomplished via currently defined Query/Retrieve SOP classes. Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 13 m

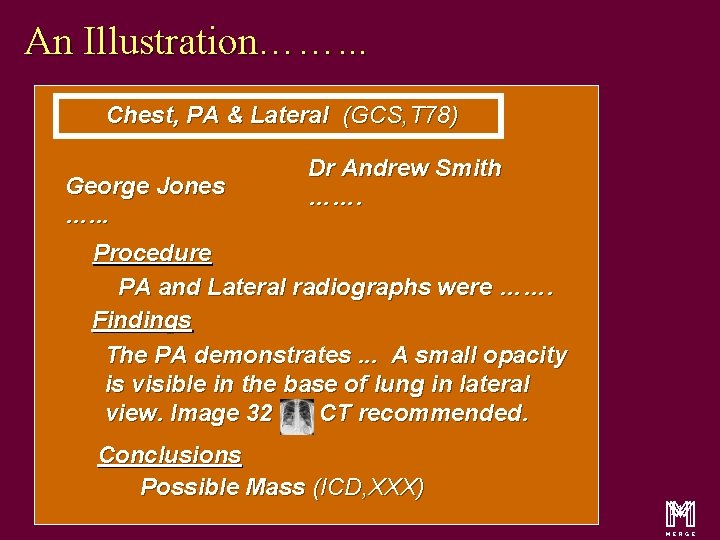
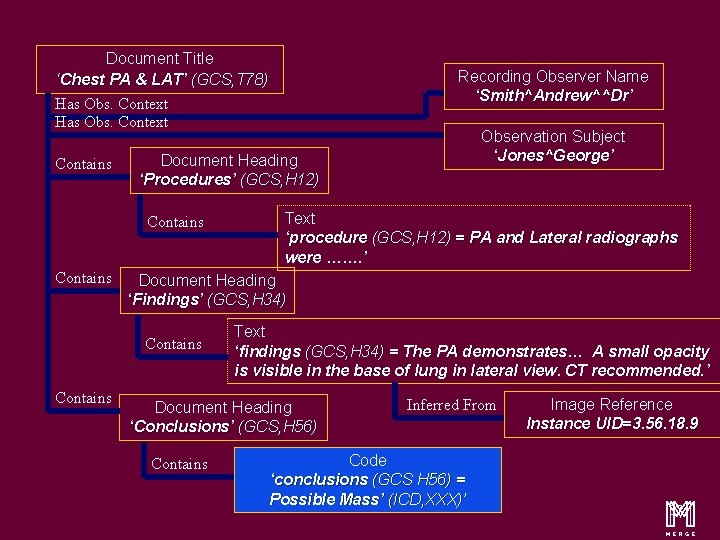
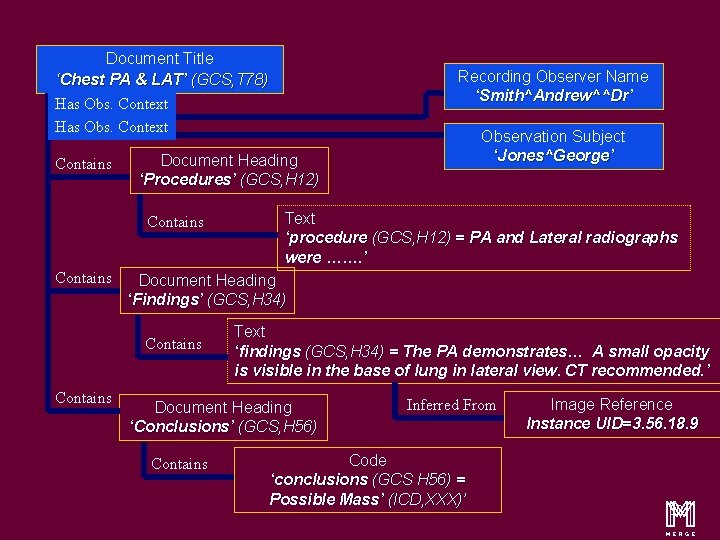
An Illustration……. . . Chest, PA & Lateral (GCS, T 78) George Jones …. . . Dr Andrew Smith ……. Procedure PA and Lateral radiographs were ……. Findings The PA demonstrates. . . A small opacity is visible in the base of lung in lateral view. Image 32 CT recommended. Conclusions Possible Mass (ICD, XXX) m 14

How do I build the SR document? u Content Items u Relationships Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 15 m

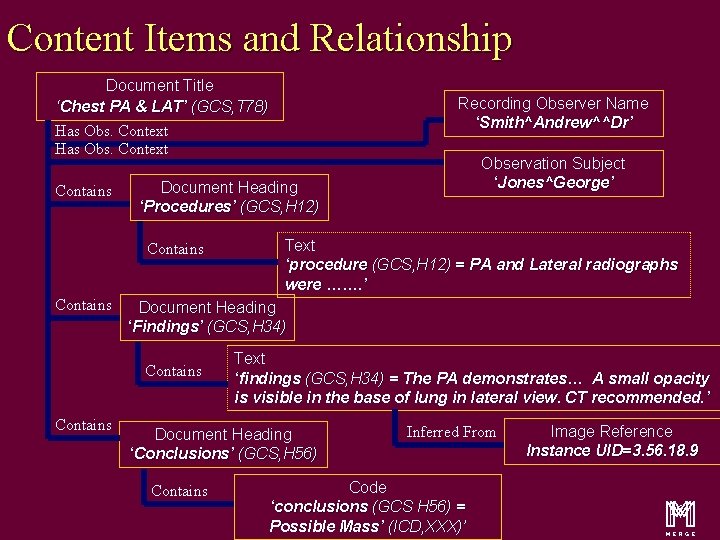
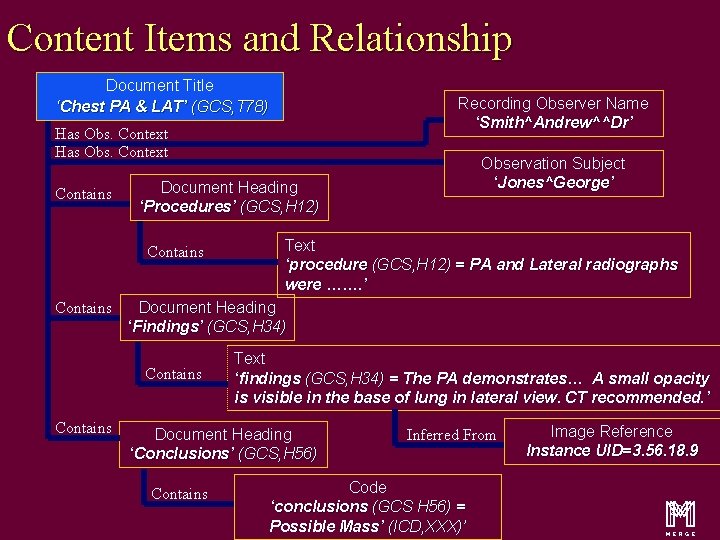
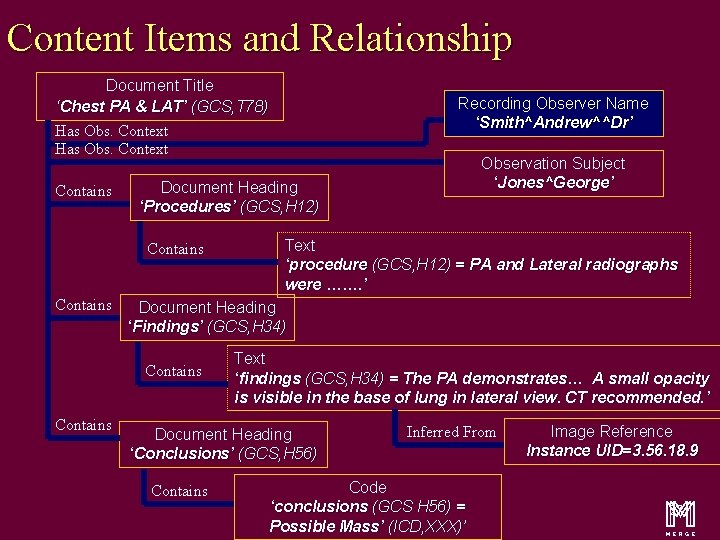
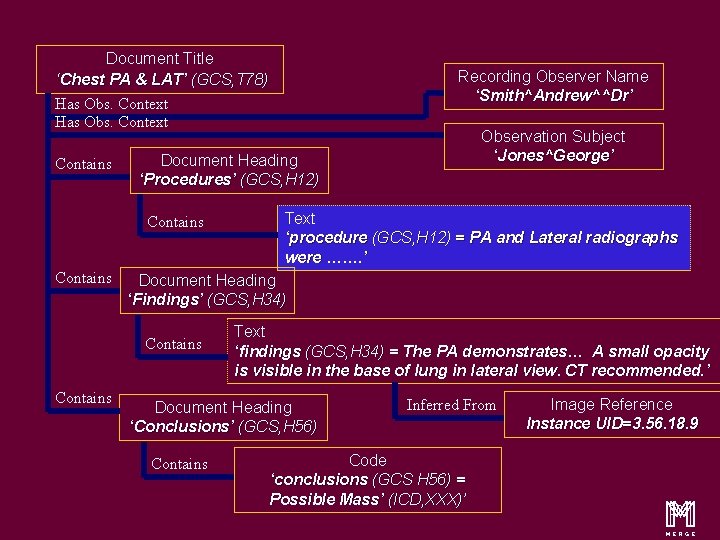
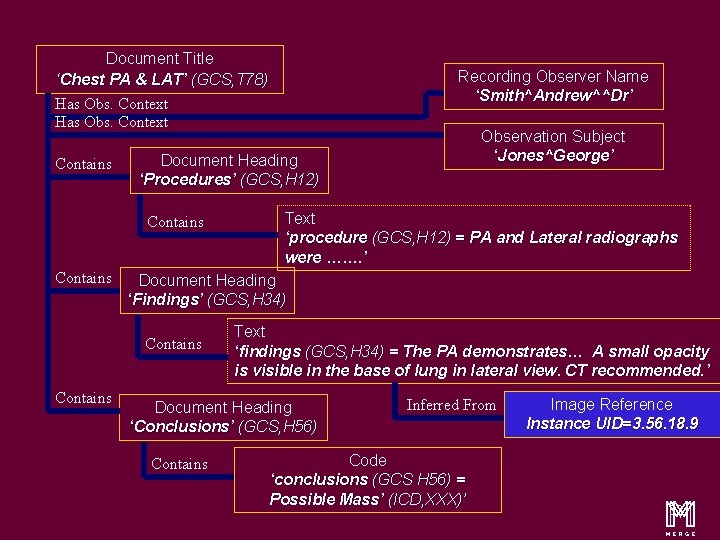
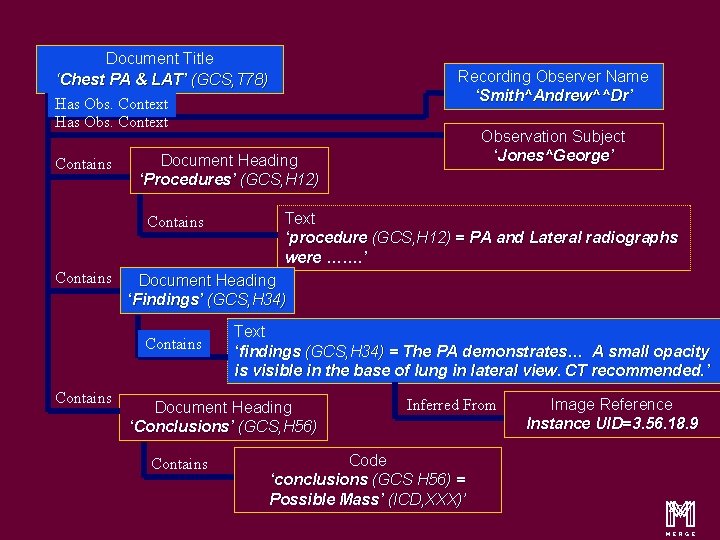
Content Items and Relationship Document Title ‘Chest PA & LAT’ (GCS, T 78) Has Obs. Context Contains Text ‘procedure (GCS, H 12) = PA and Lateral radiographs were ……. ’ Document Heading ‘Findings’ (GCS, H 34) Contains Observation Subject ‘Jones^George’ Document Heading ‘Procedures’ (GCS, H 12) Contains Recording Observer Name ‘Smith^Andrew^^Dr’ Text ‘findings (GCS, H 34) = The PA demonstrates… A small opacity is visible in the base of lung in lateral view. CT recommended. ’ Document Heading ‘Conclusions’ (GCS, H 56) Contains Inferred From Code ‘conclusions (GCS H 56) = Possible Mass’ (ICD, XXX)’ Image Reference Instance UID=3. 56. 18. 9 m 16

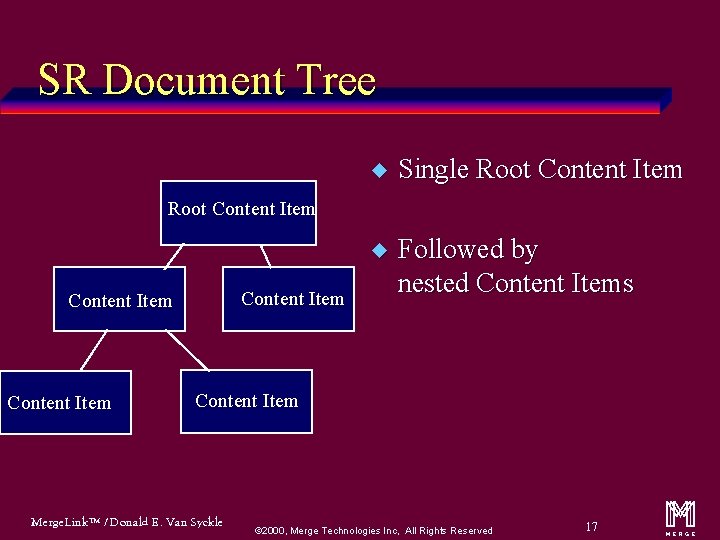
SR Document Tree u Single Root Content Item u Followed by nested Content Items Root Content Item Content Item Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 17 m

Content Items Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 18 m

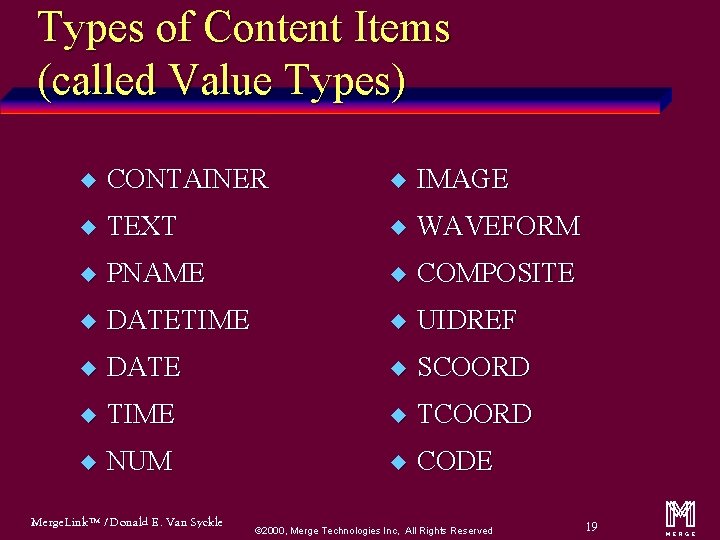
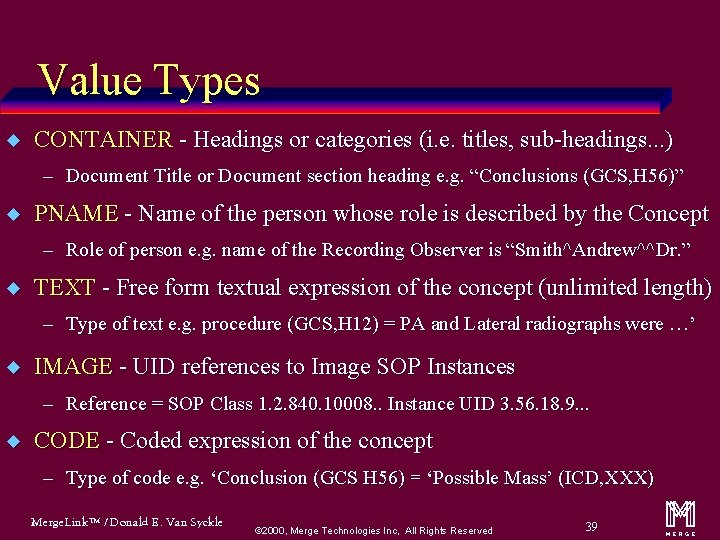
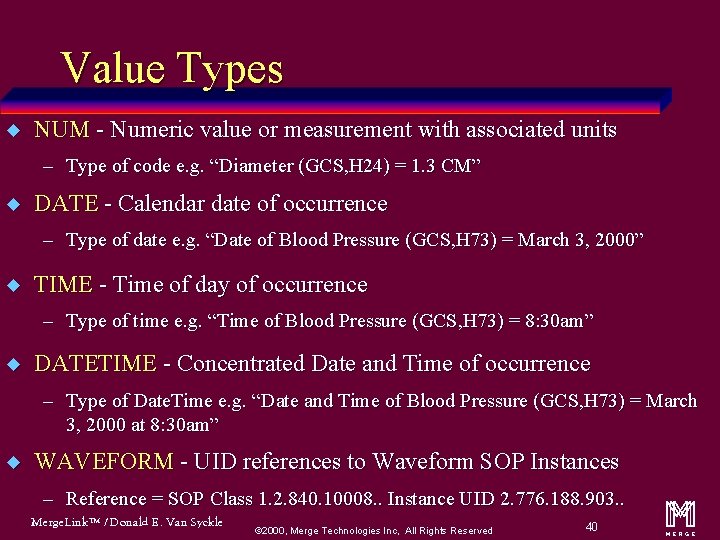
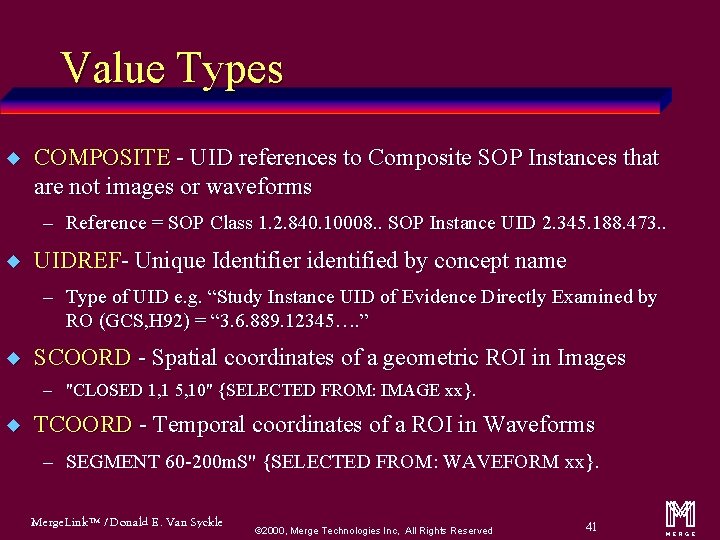
Types of Content Items (called Value Types) u CONTAINER u IMAGE u TEXT u WAVEFORM u PNAME u COMPOSITE u DATETIME u UIDREF u DATE u SCOORD u TIME u TCOORD u NUM u CODE Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 19 m

Content Items and Relationship Document Title ‘Chest PA & LAT’ (GCS, T 78) Recording Observer Name ‘Smith^Andrew^^Dr’ Has Obs. Context Contains Document Heading ‘Procedures’ (GCS, H 12) Contains Text ‘procedure (GCS, H 12) = PA and Lateral radiographs were ……. ’ Document Heading ‘Findings’ (GCS, H 34) Contains Observation Subject ‘Jones^George’ Text ‘findings (GCS, H 34) = The PA demonstrates… A small opacity is visible in the base of lung in lateral view. CT recommended. ’ Document Heading ‘Conclusions’ (GCS, H 56) Contains Inferred From Code ‘conclusions (GCS H 56) = Possible Mass’ (ICD, XXX)’ Image Reference Instance UID=3. 56. 18. 9 m 20

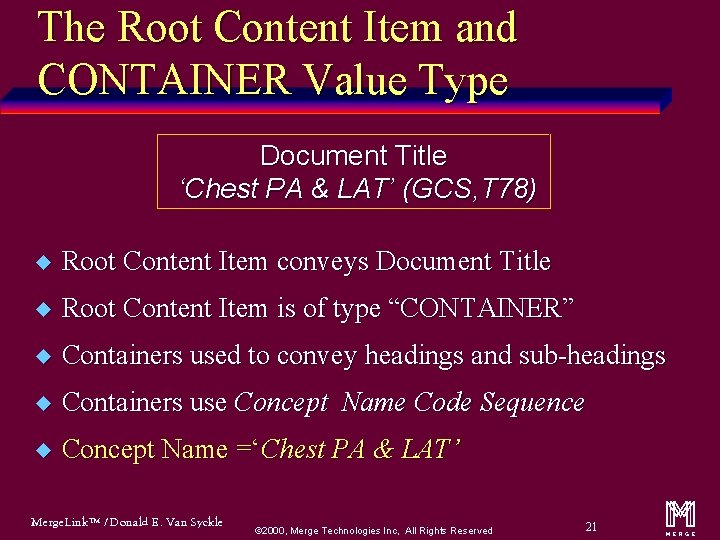
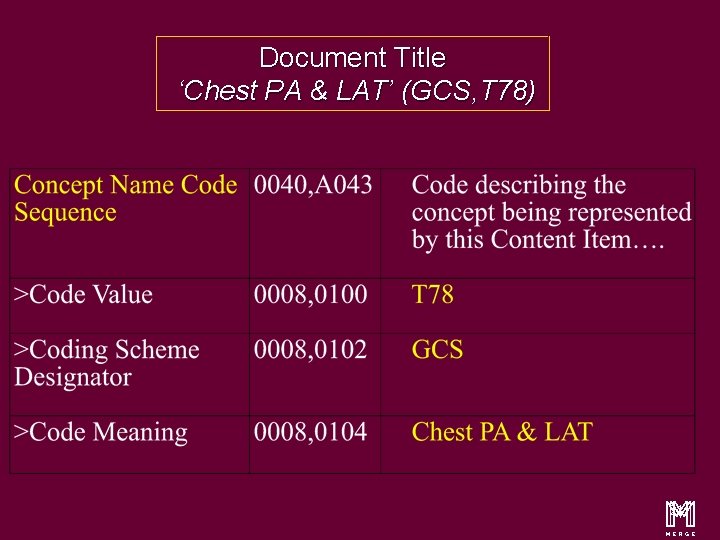
The Root Content Item and CONTAINER Value Type Document Title ‘Chest PA & LAT’ (GCS, T 78) u Root Content Item conveys Document Title u Root Content Item is of type “CONTAINER” u Containers used to convey headings and sub-headings u Containers use Concept Name Code Sequence u Concept Name =‘Chest PA & LAT’ Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 21 m

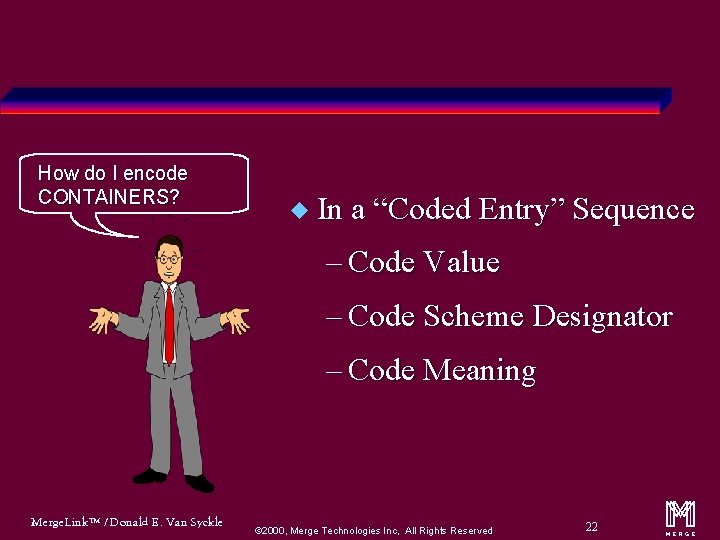
How do I encode CONTAINERS? u In a “Coded Entry” Sequence – Code Value – Code Scheme Designator – Code Meaning Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 22 m

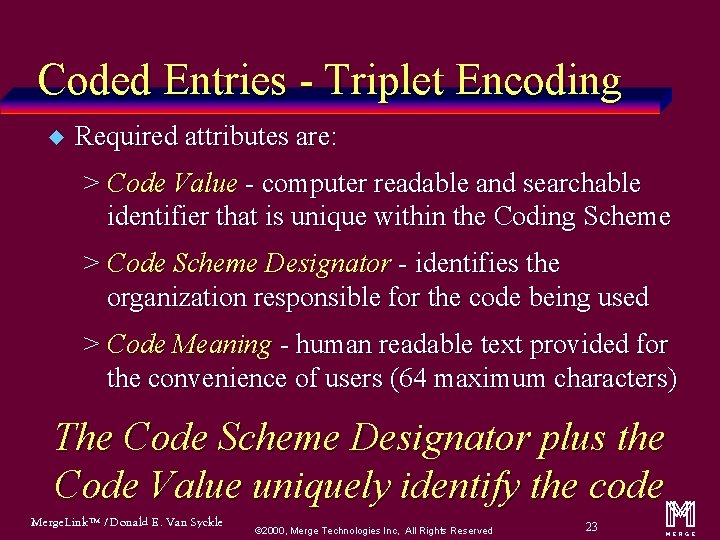
Coded Entries - Triplet Encoding u Required attributes are: > Code Value - computer readable and searchable identifier that is unique within the Coding Scheme > Code Scheme Designator - identifies the organization responsible for the code being used > Code Meaning - human readable text provided for the convenience of users (64 maximum characters) The Code Scheme Designator plus the Code Value uniquely identify the code Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 23 m

Document Title ‘Chest PA & LAT’ (GCS, T 78) m 24

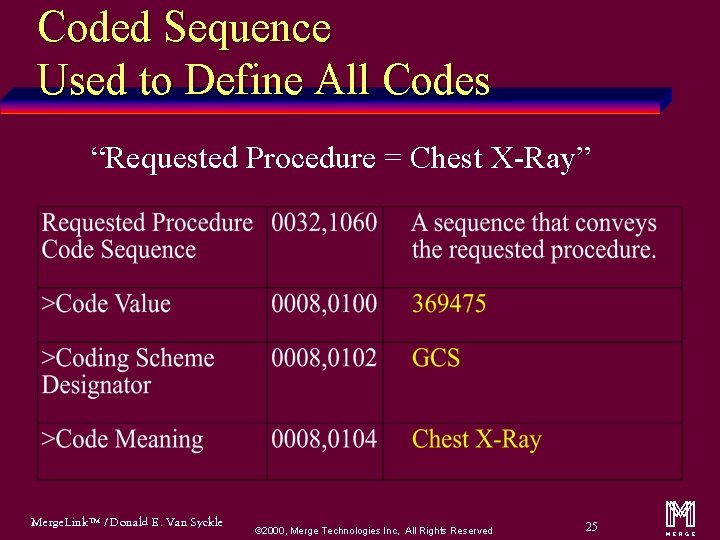
Coded Sequence Used to Define All Codes “Requested Procedure = Chest X-Ray” Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 25 m

DICOM Defines the Ability To: u More precisely define the Coding Scheme Designator u Extend existing Coding Schemes u Define “Private Coding Schemes” Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 26 m

Content Items and Relationship Document Title ‘Chest PA & LAT’ (GCS, T 78) Has Obs. Context Contains Text ‘procedure (GCS, H 12) = PA and Lateral radiographs were ……. ’ Document Heading ‘Findings’ (GCS, H 34) Contains Observation Subject ‘Jones^George’ Document Heading ‘Procedures’ (GCS, H 12) Contains Recording Observer Name ‘Smith^Andrew^^Dr’ Text ‘findings (GCS, H 34) = The PA demonstrates… A small opacity is visible in the base of lung in lateral view. CT recommended. ’ Document Heading ‘Conclusions’ (GCS, H 56) Contains Inferred From Code ‘conclusions (GCS H 56) = Possible Mass’ (ICD, XXX)’ Image Reference Instance UID=3. 56. 18. 9 m 27

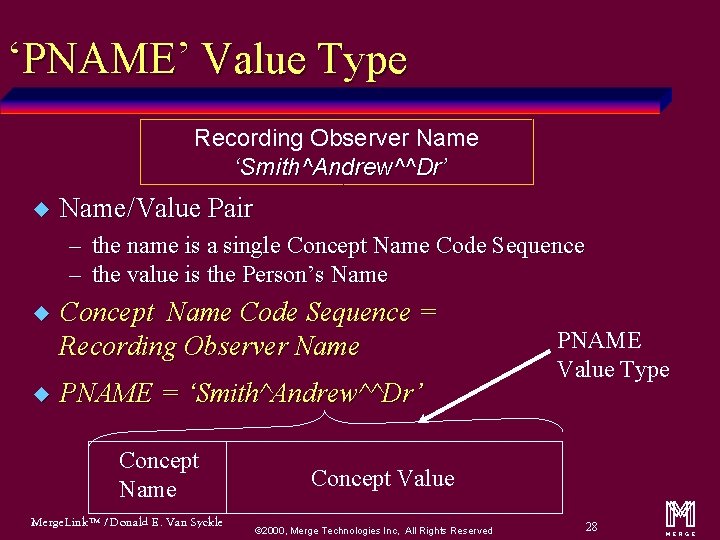
‘PNAME’ Value Type Recording Observer Name ‘Smith^Andrew^^Dr’ u Name/Value Pair – the name is a single Concept Name Code Sequence – the value is the Person’s Name u u Concept Name Code Sequence = Recording Observer Name PNAME = ‘Smith^Andrew^^Dr’ Concept Name Merge. Link™ / Donald E. Van Syckle PNAME Value Type Concept Value ã 2000, Merge Technologies Inc, All Rights Reserved 28 m

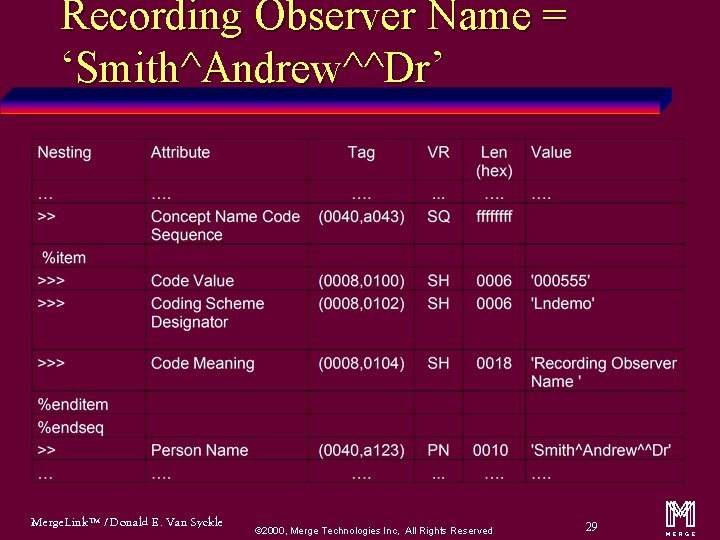
Recording Observer Name = ‘Smith^Andrew^^Dr’ Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 29 m

Document Title ‘Chest PA & LAT’ (GCS, T 78) Has Obs. Context Contains Text ‘procedure (GCS, H 12) = PA and Lateral radiographs were ……. ’ Document Heading ‘Findings’ (GCS, H 34) Contains Observation Subject ‘Jones^George’ Document Heading ‘Procedures’ (GCS, H 12) Contains Recording Observer Name ‘Smith^Andrew^^Dr’ Text ‘findings (GCS, H 34) = The PA demonstrates… A small opacity is visible in the base of lung in lateral view. CT recommended. ’ Document Heading ‘Conclusions’ (GCS, H 56) Contains Inferred From Code ‘conclusions (GCS H 56) = Possible Mass’ (ICD, XXX)’ Image Reference Instance UID=3. 56. 18. 9 m 30

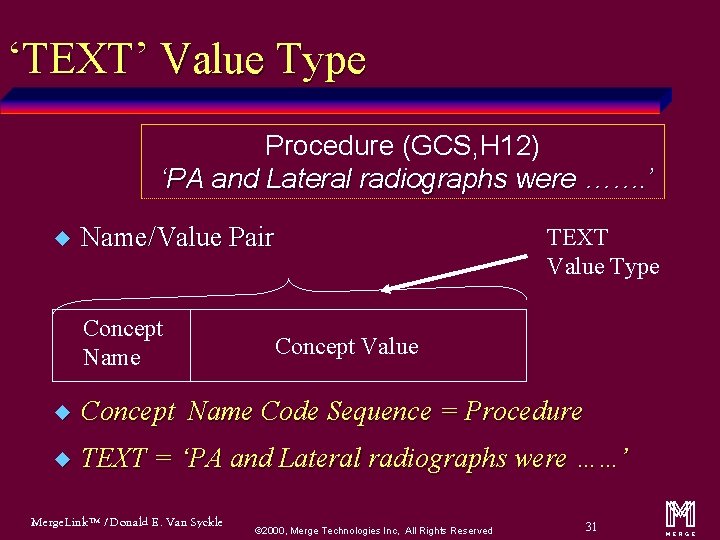
‘TEXT’ Value Type Procedure (GCS, H 12) ‘PA and Lateral radiographs were ……. ’ u Name/Value Pair Concept Name TEXT Value Type Concept Value u Concept Name Code Sequence = Procedure u TEXT = ‘PA and Lateral radiographs were …. . . ’ Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 31 m

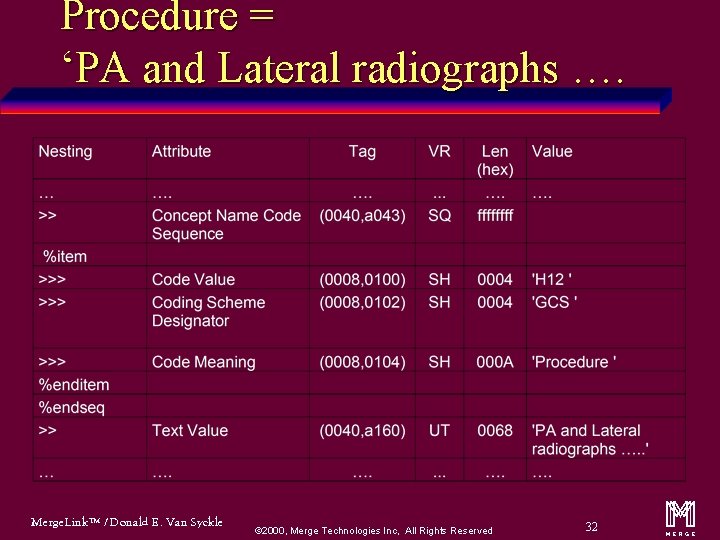
Procedure = ‘PA and Lateral radiographs …. Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 32 m

Document Title ‘Chest PA & LAT’ (GCS, T 78) Has Obs. Context Contains Text ‘procedure (GCS, H 12) = PA and Lateral radiographs were ……. ’ Document Heading ‘Findings’ (GCS, H 34) Contains Observation Subject ‘Jones^George’ Document Heading ‘Procedures’ (GCS, H 12) Contains Recording Observer Name ‘Smith^Andrew^^Dr’ Text ‘findings (GCS, H 34) = The PA demonstrates… A small opacity is visible in the base of lung in lateral view. CT recommended. ’ Document Heading ‘Conclusions’ (GCS, H 56) Contains Inferred From Code ‘conclusions (GCS H 56) = Possible Mass’ (ICD, XXX)’ Image Reference Instance UID=3. 56. 18. 9 m 33

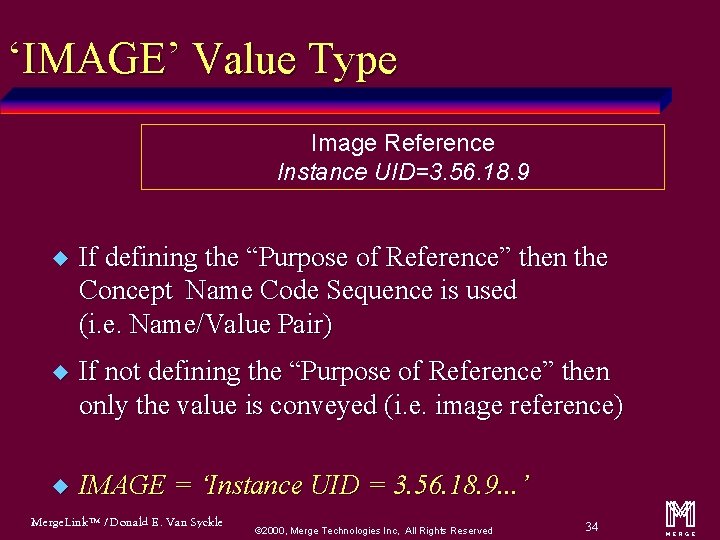
‘IMAGE’ Value Type Image Reference Instance UID=3. 56. 18. 9 u If defining the “Purpose of Reference” then the Concept Name Code Sequence is used (i. e. Name/Value Pair) u If not defining the “Purpose of Reference” then only the value is conveyed (i. e. image reference) u IMAGE = ‘Instance UID = 3. 56. 18. 9. . . ’ Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 34 m

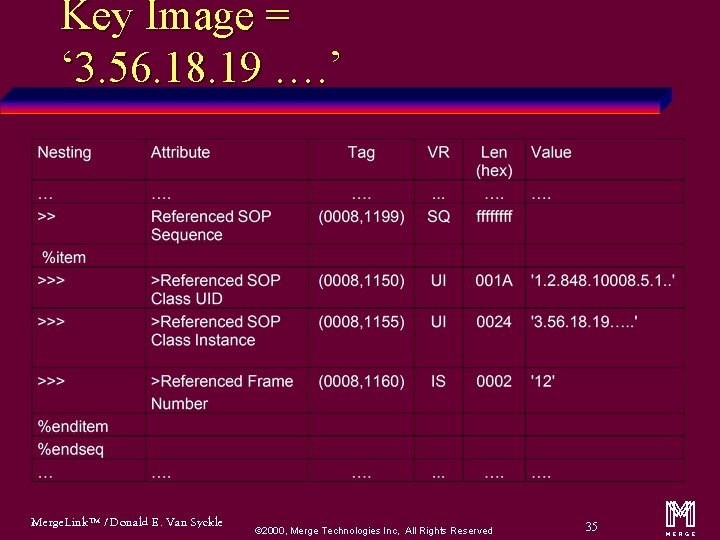
Key Image = ‘ 3. 56. 18. 19 …. ’ Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 35 m

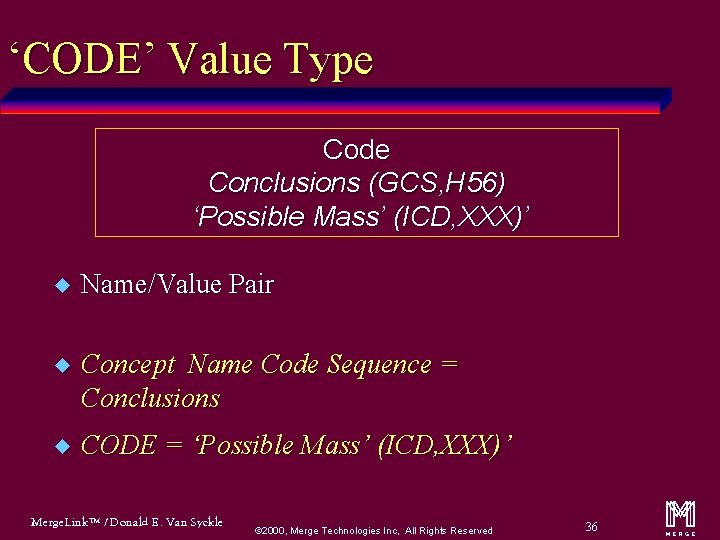
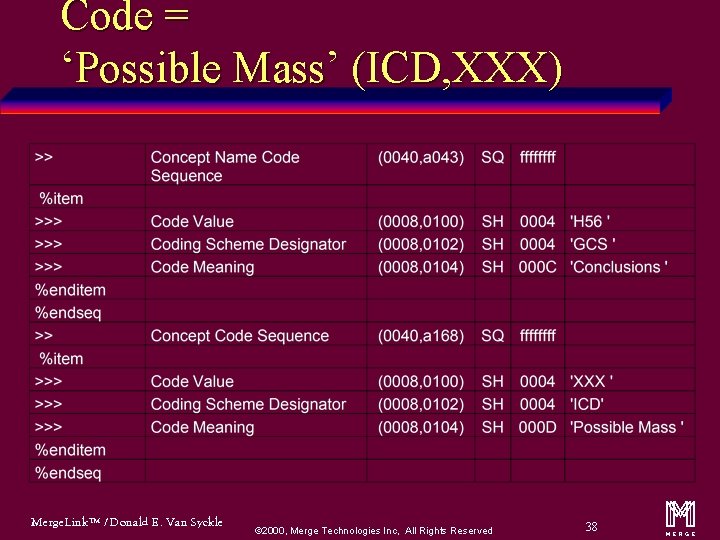
‘CODE’ Value Type Code Conclusions (GCS, H 56) ‘Possible Mass’ (ICD, XXX)’ u Name/Value Pair u Concept Name Code Sequence = Conclusions u CODE = ‘Possible Mass’ (ICD, XXX)’ Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 36 m

Document Title ‘Chest PA & LAT’ (GCS, T 78) Has Obs. Context Contains Text ‘procedure (GCS, H 12) = PA and Lateral radiographs were ……. ’ Document Heading ‘Findings’ (GCS, H 34) Contains Observation Subject ‘Jones^George’ Document Heading ‘Procedures’ (GCS, H 12) Contains Recording Observer Name ‘Smith^Andrew^^Dr’ Text ‘findings (GCS, H 34) = The PA demonstrates… A small opacity is visible in the base of lung in lateral view. CT recommended. ’ Document Heading ‘Conclusions’ (GCS, H 56) Contains Inferred From Code ‘conclusions (GCS H 56) = Possible Mass’ (ICD, XXX)’ Image Reference Instance UID=3. 56. 18. 9 m 37

Code = ‘Possible Mass’ (ICD, XXX) Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 38 m

Value Types u CONTAINER - Headings or categories (i. e. titles, sub-headings. . . ) – Document Title or Document section heading e. g. “Conclusions (GCS, H 56)” u PNAME - Name of the person whose role is described by the Concept – Role of person e. g. name of the Recording Observer is “Smith^Andrew^^Dr. ” u TEXT - Free form textual expression of the concept (unlimited length) – Type of text e. g. procedure (GCS, H 12) = PA and Lateral radiographs were …’ u IMAGE - UID references to Image SOP Instances – Reference = SOP Class 1. 2. 840. 10008. . Instance UID 3. 56. 18. 9. . . u CODE - Coded expression of the concept – Type of code e. g. ‘Conclusion (GCS H 56) = ‘Possible Mass’ (ICD, XXX) Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 39 m

Value Types u NUM - Numeric value or measurement with associated units – Type of code e. g. “Diameter (GCS, H 24) = 1. 3 CM” u DATE - Calendar date of occurrence – Type of date e. g. “Date of Blood Pressure (GCS, H 73) = March 3, 2000” u TIME - Time of day of occurrence – Type of time e. g. “Time of Blood Pressure (GCS, H 73) = 8: 30 am” u DATETIME - Concentrated Date and Time of occurrence – Type of Date. Time e. g. “Date and Time of Blood Pressure (GCS, H 73) = March 3, 2000 at 8: 30 am” u WAVEFORM - UID references to Waveform SOP Instances – Reference = SOP Class 1. 2. 840. 10008. . Instance UID 2. 776. 188. 903. . Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 40 m

Value Types u COMPOSITE - UID references to Composite SOP Instances that are not images or waveforms – Reference = SOP Class 1. 2. 840. 10008. . SOP Instance UID 2. 345. 188. 473. . u UIDREF- Unique Identifier identified by concept name – Type of UID e. g. “Study Instance UID of Evidence Directly Examined by RO (GCS, H 92) = “ 3. 6. 889. 12345…. ” u SCOORD - Spatial coordinates of a geometric ROI in Images – "CLOSED 1, 1 5, 10" {SELECTED FROM: IMAGE xx}. u TCOORD - Temporal coordinates of a ROI in Waveforms – SEGMENT 60 -200 m. S" {SELECTED FROM: WAVEFORM xx}. Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 41 m

How are the Content Items linked together in the SR Tree? u Relationship Types Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 42 m

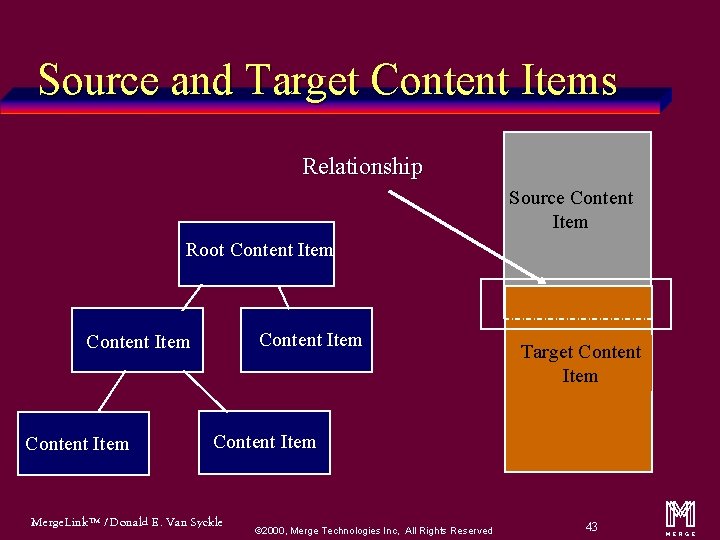
Source and Target Content Items Relationship Source Content Item Root Content Item Target Content Item Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 43 m

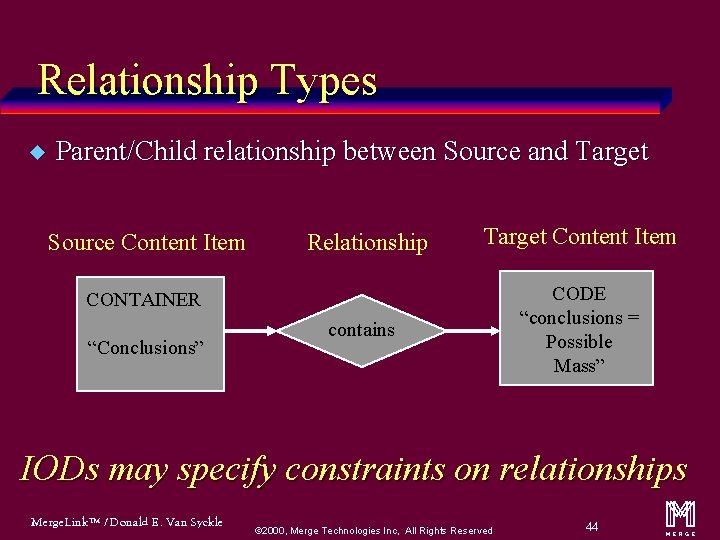
Relationship Types u Parent/Child relationship between Source and Target Source Content Item Relationship Target Content Item CONTAINER “Conclusions” contains CODE “conclusions = Possible Mass” IODs may specify constraints on relationships Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 44 m

Document Title ‘Chest PA & LAT’ (GCS, T 78) Has Obs. Context Contains Text ‘procedure (GCS, H 12) = PA and Lateral radiographs were ……. ’ Document Heading ‘Findings’ (GCS, H 34) Contains Observation Subject ‘Jones^George’ Document Heading ‘Procedures’ (GCS, H 12) Contains Recording Observer Name ‘Smith^Andrew^^Dr’ Text ‘findings (GCS, H 34) = The PA demonstrates… A small opacity is visible in the base of lung in lateral view. CT recommended. ’ Document Heading ‘Conclusions’ (GCS, H 56) Contains Inferred From Code ‘conclusions (GCS H 56) = Possible Mass’ (ICD, XXX)’ Image Reference Instance UID=3. 56. 18. 9 m 45

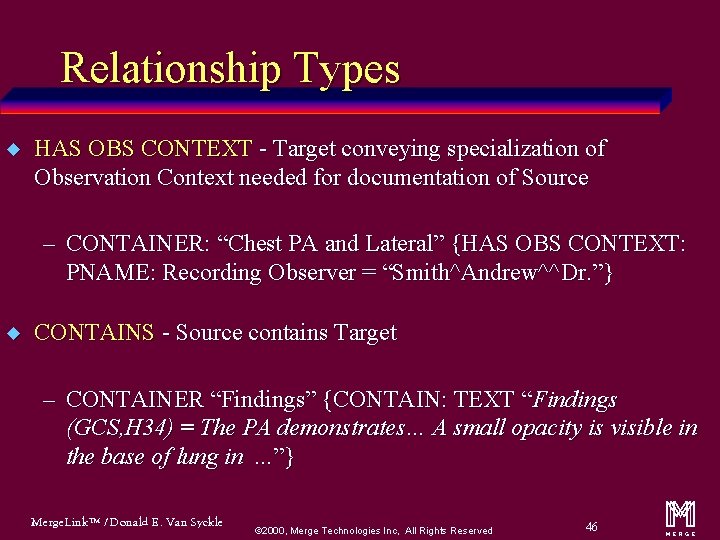
Relationship Types u HAS OBS CONTEXT - Target conveying specialization of Observation Context needed for documentation of Source – CONTAINER: “Chest PA and Lateral” {HAS OBS CONTEXT: PNAME: Recording Observer = “Smith^Andrew^^Dr. ”} u CONTAINS - Source contains Target – CONTAINER “Findings” {CONTAIN: TEXT “Findings (GCS, H 34) = The PA demonstrates… A small opacity is visible in the base of lung in. . . ”} Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 46 m

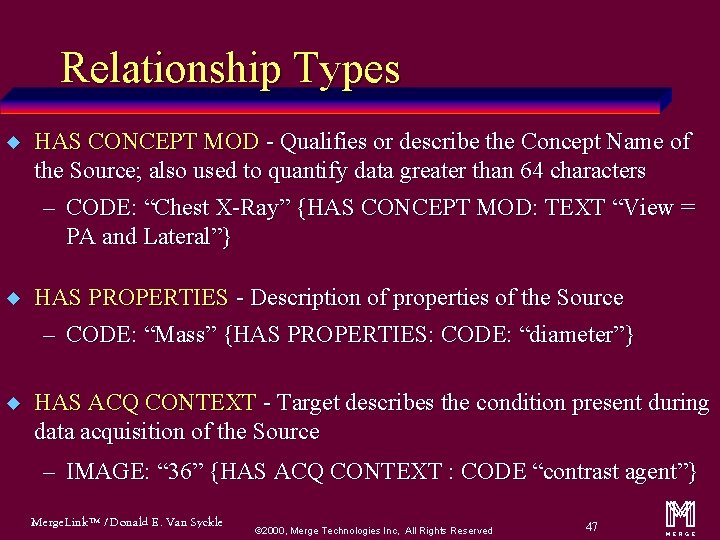
Relationship Types u HAS CONCEPT MOD - Qualifies or describe the Concept Name of the Source; also used to quantify data greater than 64 characters – CODE: “Chest X-Ray” {HAS CONCEPT MOD: TEXT “View = PA and Lateral”} u HAS PROPERTIES - Description of properties of the Source – CODE: “Mass” {HAS PROPERTIES: CODE: “diameter”} u HAS ACQ CONTEXT - Target describes the condition present during data acquisition of the Source – IMAGE: “ 36” {HAS ACQ CONTEXT : CODE “contrast agent”} Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 47 m

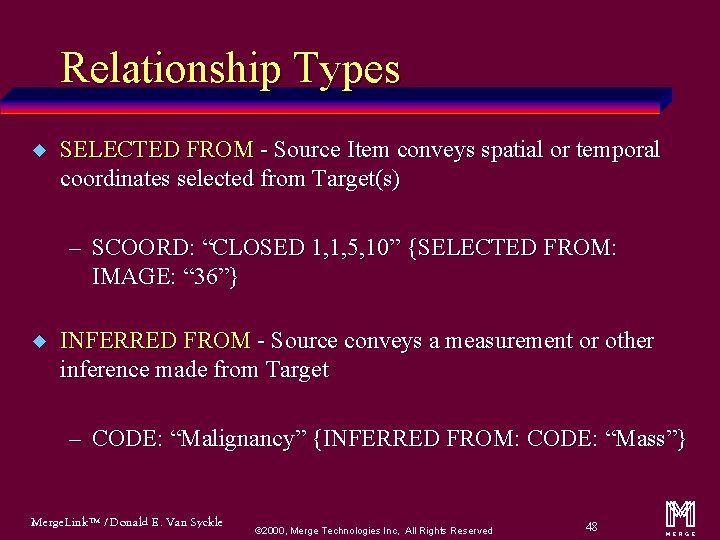
Relationship Types u SELECTED FROM - Source Item conveys spatial or temporal coordinates selected from Target(s) – SCOORD: “CLOSED 1, 1, 5, 10” {SELECTED FROM: IMAGE: “ 36”} u INFERRED FROM - Source conveys a measurement or other inference made from Target – CODE: “Malignancy” {INFERRED FROM: CODE: “Mass”} Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 48 m

Observation Context u Describes who or what is performing the interpretations u Whether the examination of evidence is direct or quoted u What procedure generated the evidence that is being interpreted u Who or what is the subject of the evidence that is being interpreted Observation Context is encoded in a Content Item Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 49 m

Document Title ‘Chest PA & LAT’ (GCS, T 78) Has Obs. Context Contains Text ‘procedure (GCS, H 12) = PA and Lateral radiographs were ……. ’ Document Heading ‘Findings’ (GCS, H 34) Contains Observation Subject ‘Jones^George’ Document Heading ‘Procedures’ (GCS, H 12) Contains Recording Observer Name ‘Smith^Andrew^^Dr’ Text ‘findings (GCS, H 34) = The PA demonstrates… A small opacity is visible in the base of lung in lateral view. CT recommended. ’ Document Heading ‘Conclusions’ (GCS, H 56) Contains Inferred From Code ‘conclusions (GCS H 56) = Possible Mass’ (ICD, XXX)’ Image Reference Instance UID=3. 56. 18. 9 m 50

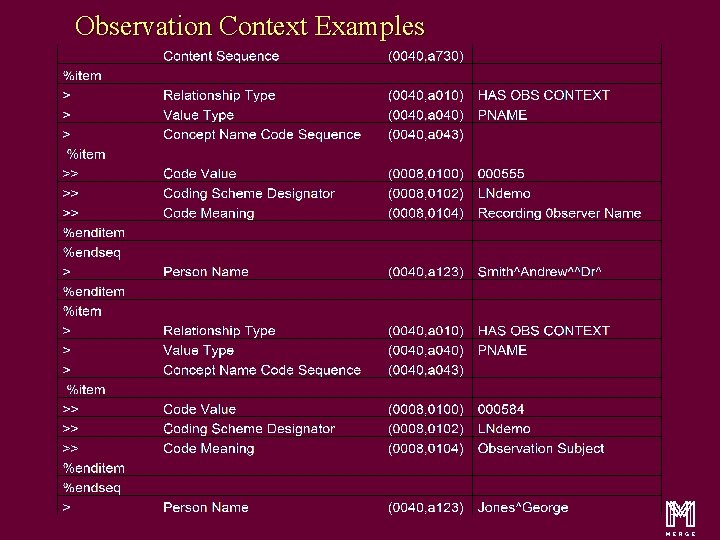
Observation Context Examples m 51

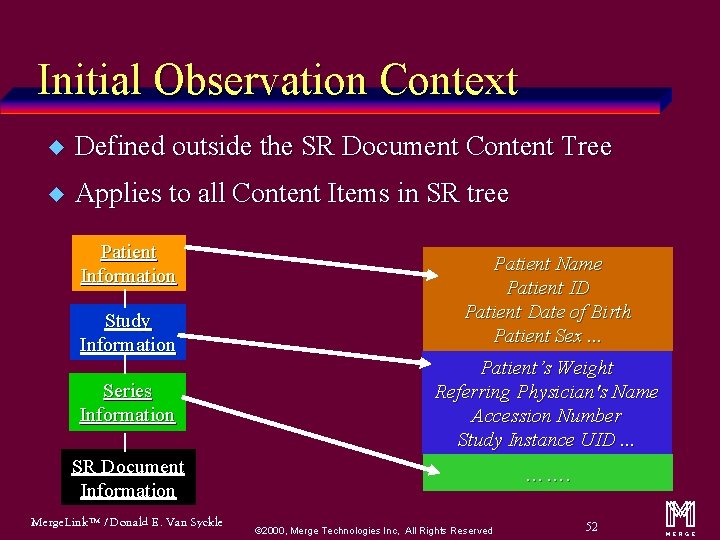
Initial Observation Context u Defined outside the SR Document Content Tree u Applies to all Content Items in SR tree Patient Information Study Information Patient Name Patient ID Patient Date of Birth Patient Sex. . . Series Information Patient’s Weight Referring Physician's Name Accession Number Study Instance UID. . . SR Document Information ……. Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 52 m

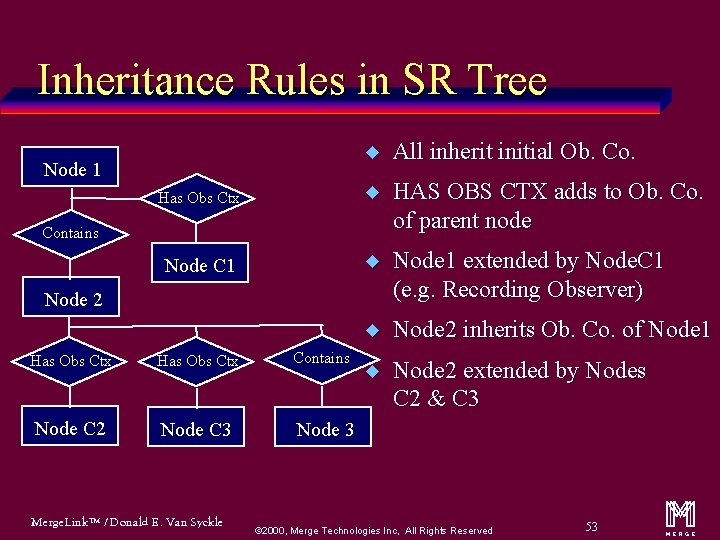
Inheritance Rules in SR Tree u All inherit initial Ob. Co. Has Obs Ctx u HAS OBS CTX adds to Ob. Co. of parent node Node C 1 u Node 1 extended by Node. C 1 (e. g. Recording Observer) u Node 2 inherits Ob. Co. of Node 1 u Node 2 extended by Nodes C 2 & C 3 Node 1 Contains Node 2 Has Obs Ctx Contains Node C 2 Node C 3 Node 3 Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 53 m

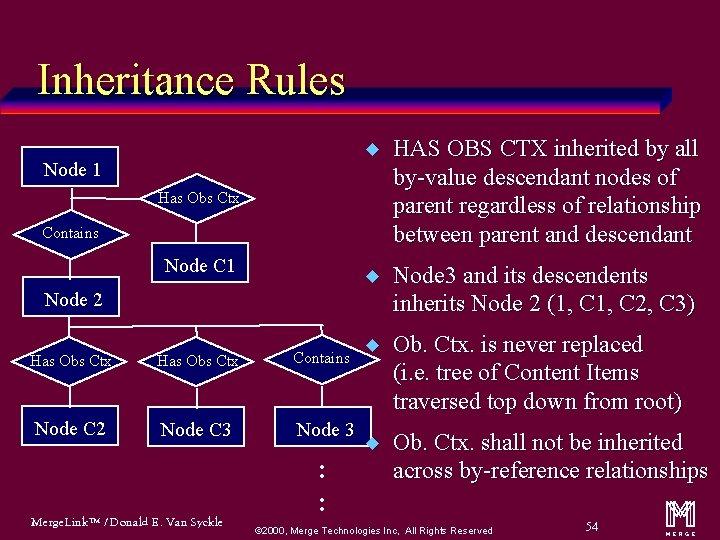
Inheritance Rules Node 1 u HAS OBS CTX inherited by all by-value descendant nodes of parent regardless of relationship between parent and descendant u Node 3 and its descendents inherits Node 2 (1, C 2, C 3) u Ob. Ctx. is never replaced (i. e. tree of Content Items traversed top down from root) u Ob. Ctx. shall not be inherited across by-reference relationships Has Obs Ctx Contains Node C 1 Node 2 Has Obs Ctx Contains Node C 2 Node C 3 Node 3 Merge. Link™ / Donald E. Van Syckle : : ã 2000, Merge Technologies Inc, All Rights Reserved 54 m

By Reference Content Items u Allows referencing to other Content Items u Document Relationship and Document Content Marcos are not included u For example: CODE: “Probable Malignancy” {INFERRED FROM: CODE: “Mass”} Code ‘Findings (GCS H 34) = Mass’ (ICD, XXX)’ Inferred From Merge. Link™ / Donald E. Van Syckle Code ‘conclusions (GCS H 56) = Probable Malignancy (ICD, YYY)’ ã 2000, Merge Technologies Inc, All Rights Reserved 55 m

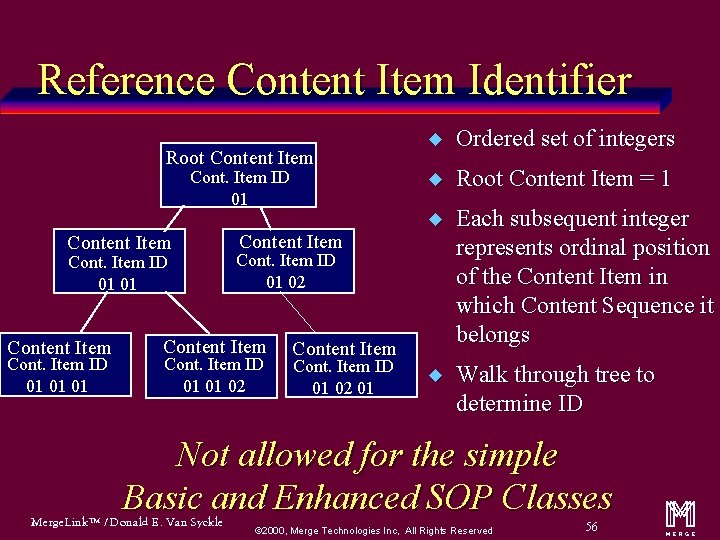
Reference Content Item Identifier Root Content Item Cont. Item ID 01 01 01 Content Item u Ordered set of integers u Root Content Item = 1 u Each subsequent integer represents ordinal position of the Content Item in which Content Sequence it belongs u Walk through tree to determine ID Cont. Item ID 01 02 Content Item Cont. Item ID 01 02 01 Not allowed for the simple Basic and Enhanced SOP Classes Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 56 m

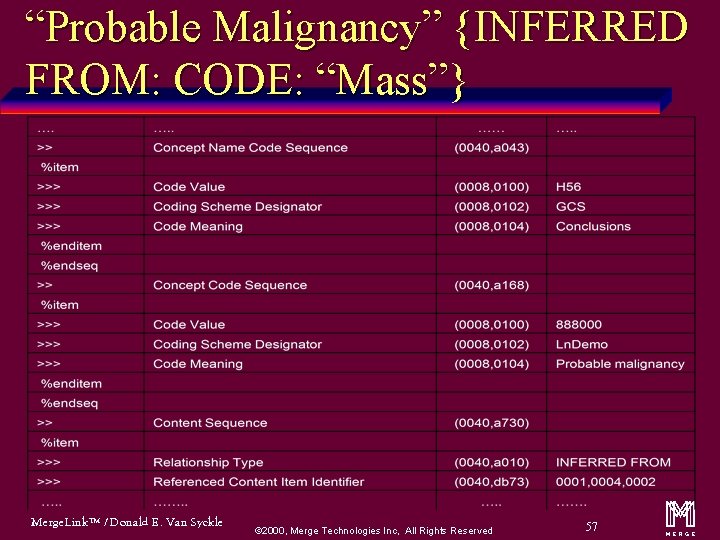
“Probable Malignancy” {INFERRED FROM: CODE: “Mass”} Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 57 m

SR Document General & SR Document Content Modules How do I read the SR specific Modules? u Same as other DICOM Modules u Except new documentation convention called “Macros” Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 58 m

Macros - a new convention for DICOM documentation. . . Easy way to document codes, measurements, spatial coordinates, image references, etc. Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 59 m

Numeric Measurement Macro Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 60 m

SR Document General Module u Attributes identify the SR Document and provide context for the entire document u Attributes are outside the SR Document Tree which simplifies Query access u See SR Supplement Table C. 17 -2 Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 61 m

Identification Attributes u SOP Class UID and SOP Instance UID (from SOP Common) u Instance Number u Content Date (same attribute as Image Date) u Content Time (same attribute as Image Time) All Type 1 Fields Date/Time important for managing reports Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 62 m

Status and Verifying Attributes u Completion Flag - PARTIAL or COMPLETE – how to set the flag is open to implementation u Completion Flag Description - free form text u Verification Flag - UNVERIFIED or VERIFIED u Verifying Observer Sequence – identifies person(s) verifying the document and organization(s); required if flag = VERIFIED Prevailing final version = most recent Verification Date. Time, VERIFIED, COMPLETE Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 63 m

Reference Request Sequence u Identifies Requested Procedure(s) being fulfilled by the SR document – Study Instance UID – Referenced Study Sequence – Requested Procedure ID, Accession Number – Requested and Performed Procedure Codes – …. One SR document may cover multiple Requested Procedures Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 64 m

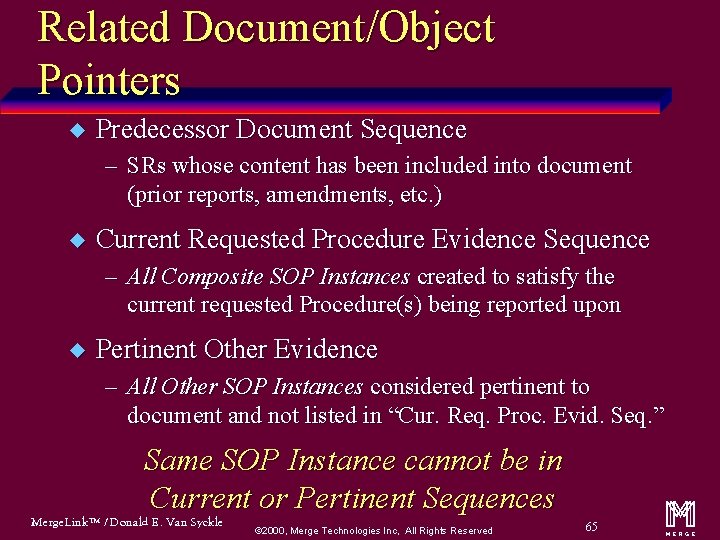
Related Document/Object Pointers u Predecessor Document Sequence – SRs whose content has been included into document (prior reports, amendments, etc. ) u Current Requested Procedure Evidence Sequence – All Composite SOP Instances created to satisfy the current requested Procedure(s) being reported upon u Pertinent Other Evidence – All Other SOP Instances considered pertinent to document and not listed in “Cur. Req. Proc. Evid. Seq. ” Same SOP Instance cannot be in Current or Pertinent Sequences Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 65 m

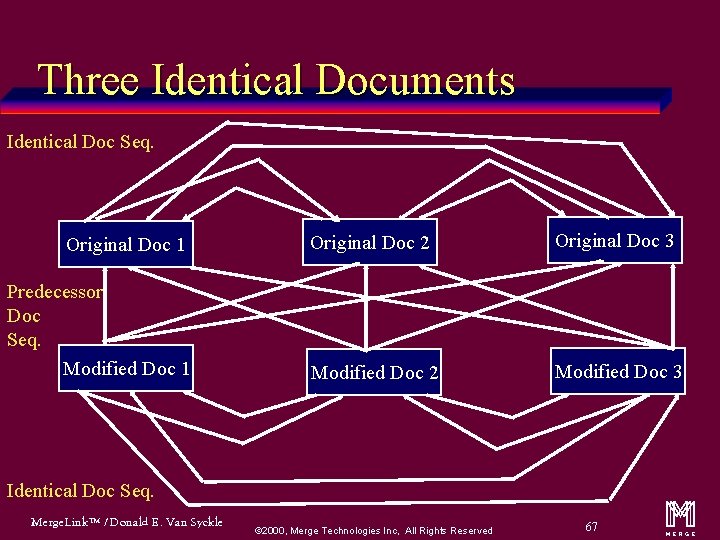
Identical Documents Sequence u References all identical SR documents u Entire SR document is the same except for UIDs (i. e. Study Instance, Series Instance, SOP Instance) u When modifying an “Identical Document” the implementation must modify all “Identical Documents” or the new “modified” report is no longer an “Identical Document” All “Identical” SR documents reference each other Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 66 m

Three Identical Documents Identical Doc Seq. Original Doc 1 Predecessor Doc Seq. Modified Doc 1 Original Doc 2 Original Doc 3 Modified Doc 2 Modified Doc 3 Identical Doc Seq. Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 67 m

SR Document Content Module u Attributes convey content of the SR Document u Provides the “Root Content Item” (i. e. Document Title) u Nests Content Sequences for all other Content Items u See SR Supplement Table C. 17. 3 – Document Relationship Macro – Document Content Macro Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 68 m

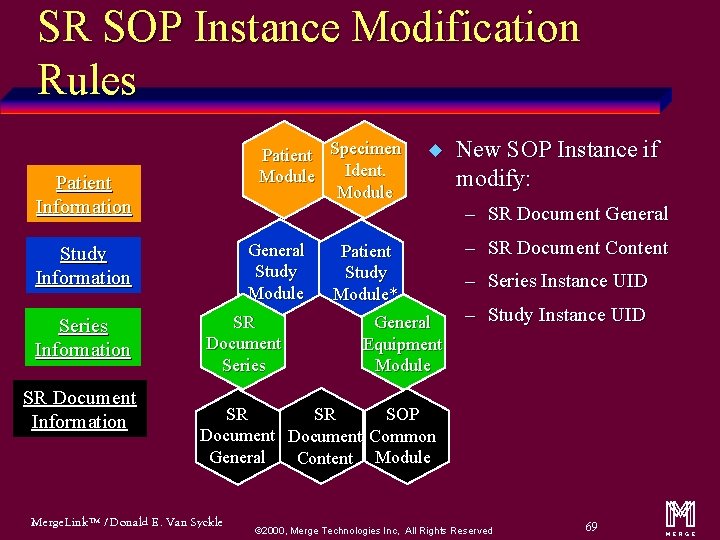
SR SOP Instance Modification Rules Patient Specimen Ident. Module Patient Information General Study Module Study Information Series Information SR Document Series u Patient Study Module* General Equipment Module New SOP Instance if modify: – SR Document General – SR Document Content – Series Instance UID – Study Instance UID SR SOP SR Document Common General Content Module Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 69 m

SR IODs u Basic Text SR IOD – Minimal usage of coded entries – Reference to SOP Instances restricted to leaves from text tree – No by-reference Content Items (i. e. by-value only) u Enhanced SR IOD – superset of Basic Text but extended to include spatial and temporal regions of interest u Comprehensive SR IOD – superset of Basic Text and Enhanced, references to leaves not restricted and includes by-reference Content Items Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 70 m

Basic Text SR Relationship Restrictions (table A. 35. 1 -2) Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 71 m

Enhanced SR Relationship Restrictions (table A. 35. 2 -2) Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 72 m

Comprehensive SR Relationship Restrictions (table A. 35. 3 -2) Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 73 m

DICOM Structured Reporting u Fits within the normal DICOM structure u New ideas such as Content Items and Relationships u Simple for Basic Text and Enhanced u More Complex for Comprehensive Not just for Radiology and Cardiology but can be used for all “ologies” Merge. Link™ / Donald E. Van Syckle ã 2000, Merge Technologies Inc, All Rights Reserved 74 m

Thank You! m 75