Determination of the hydrofluoric acid concentration in the



![The determination of [H 2 Si. F 6] or [HF] in the same bath The determination of [H 2 Si. F 6] or [HF] in the same bath](https://slidetodoc.com/presentation_image/7eb4a4b44ca3b0a49e6fa1ff774aca94/image-8.jpg)
![[H 2 Si. F 6] is determinate by the volume difference DVm. L = [H 2 Si. F 6] is determinate by the volume difference DVm. L =](https://slidetodoc.com/presentation_image/7eb4a4b44ca3b0a49e6fa1ff774aca94/image-10.jpg)
![[F-I] = [F -] + 6 [Si. F 62 -] Potentiometric Acido/Basic VEP 3 [F-I] = [F -] + 6 [Si. F 62 -] Potentiometric Acido/Basic VEP 3](https://slidetodoc.com/presentation_image/7eb4a4b44ca3b0a49e6fa1ff774aca94/image-11.jpg)



- Slides: 14

Determination of the hydrofluoric acid concentration in the solutions used for the chemical polishing of crystal. E. Brient Cristallerie de Baccarat, 20 rue des Cristalleries - 54120 Baccarat Y. Pillet, M. Vilasi LCSM UMR 755, Faculté des Sciences et techniques UHP – Nancy I Bd des Aiguillettes BP 239 - 54506 Vandoeuvre

polishing before after

INDUSTRIAL EXPECTATIONS Savings in consumption of acids Optimization of the polishing cycles : improvement of the capacity on the existing machines Control of the « strength » of the bath to avoid quality problems as stuck salts or loss of acceptable dimensional tolerances

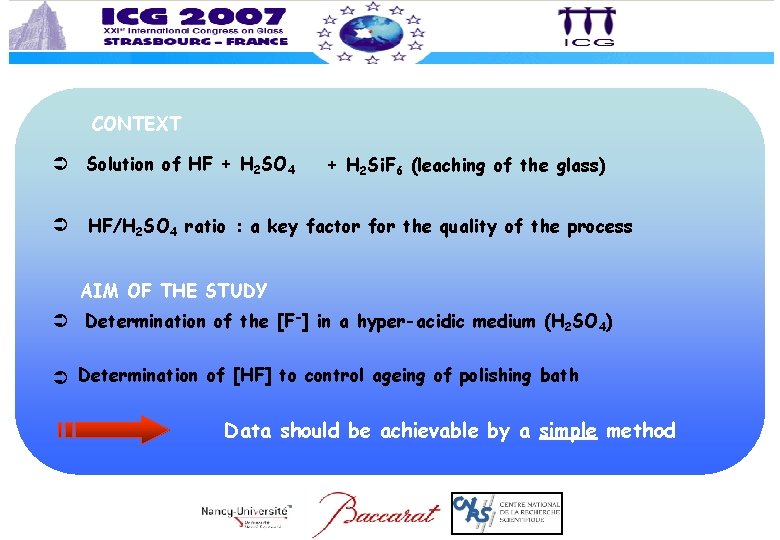
CONTEXT Solution of HF + H 2 SO 4 + H 2 Si. F 6 (leaching of the glass) HF/H 2 SO 4 ratio : a key factor for the quality of the process AIM OF THE STUDY Determination of the [F-] in a hyper-acidic medium (H 2 SO 4) Determination of [HF] to control ageing of polishing bath Data should be achievable by a simple method

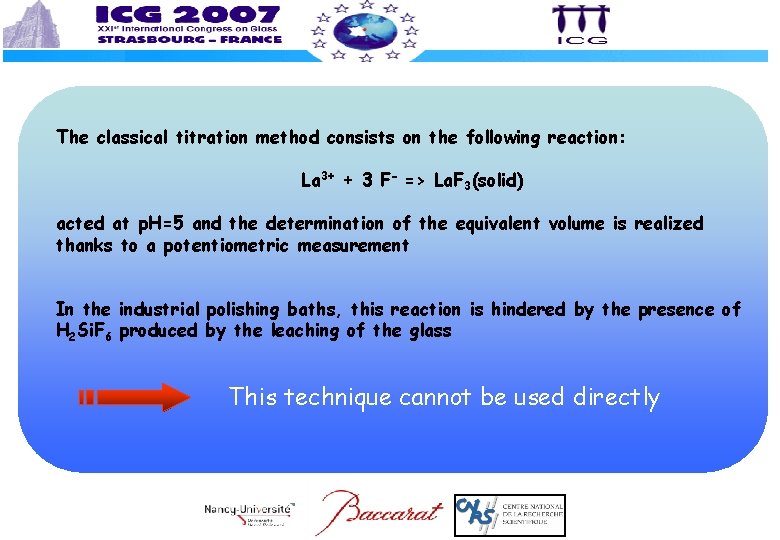
The classical titration method consists on the following reaction: La 3+ + 3 F- => La. F 3(solid) acted at p. H=5 and the determination of the equivalent volume is realized thanks to a potentiometric measurement In the industrial polishing baths, this reaction is hindered by the presence of H 2 Si. F 6 produced by the leaching of the glass This technique cannot be used directly

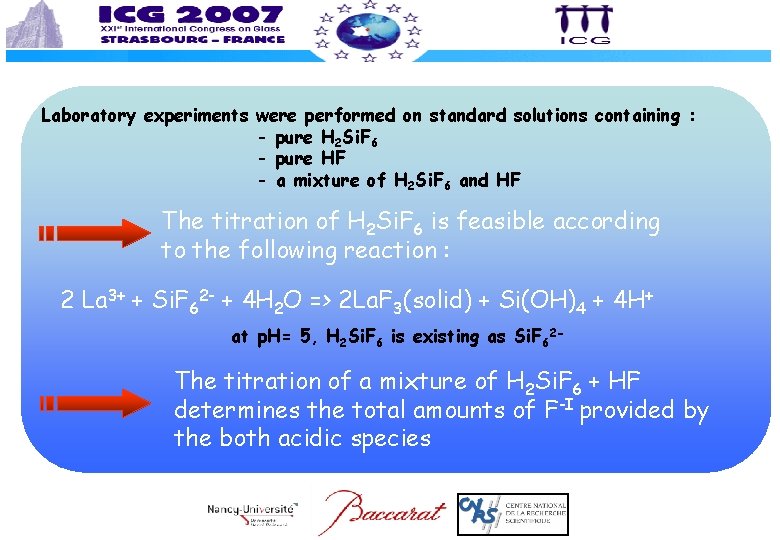
Laboratory experiments were performed on standard solutions containing : - pure H 2 Si. F 6 - pure HF - a mixture of H 2 Si. F 6 and HF The titration of H 2 Si. F 6 is feasible according to the following reaction : 2 La 3+ + Si. F 62 - + 4 H 2 O => 2 La. F 3(solid) + Si(OH)4 + 4 H+ at p. H= 5, H 2 Si. F 6 is existing as Si. F 62 - The titration of a mixture of H 2 Si. F 6 + HF determines the total amounts of F-I provided by the both acidic species

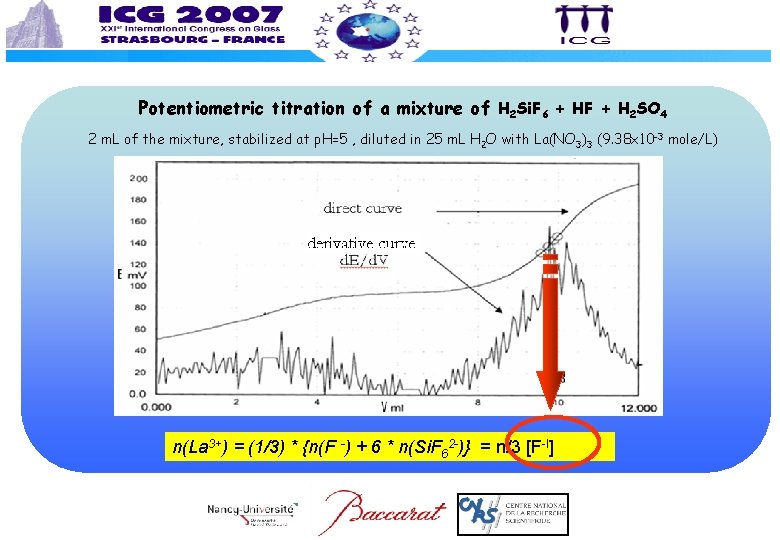
Potentiometric titration of a mixture of H 2 Si. F 6 + HF + H 2 SO 4 2 m. L of the mixture, stabilized at p. H=5 , diluted in 25 m. L H 2 O with La(NO 3)3 (9. 38 x 10 -3 mole/L) n(La 3+) = (1/3) * {n(F -) + 6 * n(Si. F 62 -)} = n/3 [F-I]
![The determination of H 2 Si F 6 or HF in the same bath The determination of [H 2 Si. F 6] or [HF] in the same bath](https://slidetodoc.com/presentation_image/7eb4a4b44ca3b0a49e6fa1ff774aca94/image-8.jpg)
The determination of [H 2 Si. F 6] or [HF] in the same bath needs to use an other technique of titration The easiest titration technique coming in mind is the acid/base method The titration of standard solutions containing H 2 Si. F 6 was studied at the laboratory

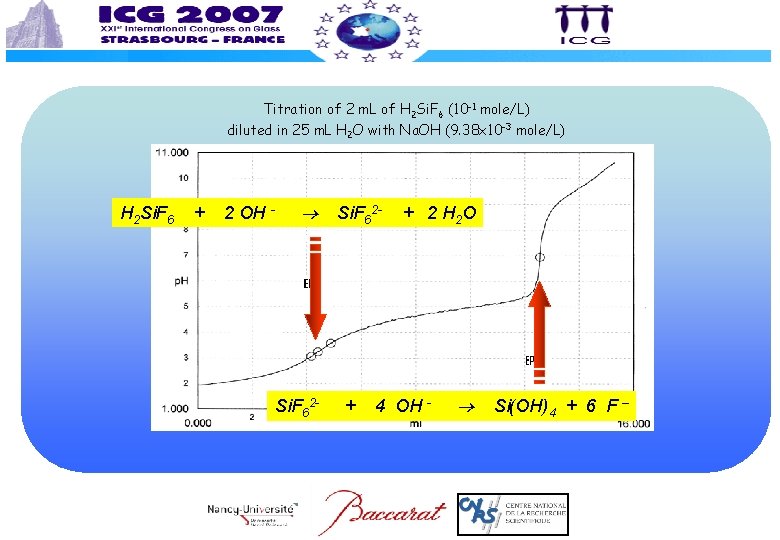
Titration of 2 m. L of H 2 Si. F 6 (10 -1 mole/L) diluted in 25 m. L H 2 O with Na. OH (9. 38 x 10 -3 mole/L) H 2 Si. F 6 + 2 OH - Si. F 62 - + + 2 H 2 O 4 OH - Si(OH)4 + 6 F –
![H 2 Si F 6 is determinate by the volume difference DVm L [H 2 Si. F 6] is determinate by the volume difference DVm. L =](https://slidetodoc.com/presentation_image/7eb4a4b44ca3b0a49e6fa1ff774aca94/image-10.jpg)
[H 2 Si. F 6] is determinate by the volume difference DVm. L = VEP 2 – VEP 1 DVm. L Titration of a standard mixture of H 2 SO 4 + HF + H 2 Si. F 6 gives a similar curve to the one obtained with pure H 2 Si. F 6 solution Technique is therefore applicable to an industrial bath
![FI F 6 Si F 62 Potentiometric AcidoBasic VEP 3 [F-I] = [F -] + 6 [Si. F 62 -] Potentiometric Acido/Basic VEP 3](https://slidetodoc.com/presentation_image/7eb4a4b44ca3b0a49e6fa1ff774aca94/image-11.jpg)
[F-I] = [F -] + 6 [Si. F 62 -] Potentiometric Acido/Basic VEP 3 DVm. L

CONCLUSIONS Chemical characterization of fluoride in hyperacidic medium can be done with an accuracy of about 10%. This method is validated on industrial solutions for crystal polishing Its simplicity allows the application on the glass making site. It should become a powerful tool to control the ageing of polishing baths

FIRST INDUSTRIAL POSITIVE CONSEQUENCE The “virtuous circle” Decrease of the ratio HF/H 2 SO 4 (by additional H 2 SO 4 and constant HF) corresponds to a lower value of H 2 Si. F 6 in the bath and lower necessary additions of HF to maintain a constant level of active HF in the bath The hypothesis to be checked is the following one: 1. Ions Si. F 62 - react better with the additional H+ than with K+ to form H 2 Si. F 6 instead of K 2 Si. F 6 2. the following reaction permits to re-create active HF: H 2 Si. F 6 + 2 H 2 O => Si. O 2 + 6 HF

 Hydrofluoric acid reaction with water
Hydrofluoric acid reaction with water Hydrofluoric acid
Hydrofluoric acid Hydrofluoric acid
Hydrofluoric acid Hydrofluoric acid
Hydrofluoric acid Whats a concentration gradient
Whats a concentration gradient Movement of high concentration to low concentration
Movement of high concentration to low concentration Strong and weak acids
Strong and weak acids Formal ether sedimentation technique
Formal ether sedimentation technique Is hcn acid or base
Is hcn acid or base 9-which acid is not considered a strong acid?
9-which acid is not considered a strong acid? Identifying lewis acids and bases practice
Identifying lewis acids and bases practice Stomach acid vs battery acid
Stomach acid vs battery acid Non acid fast bacteria
Non acid fast bacteria Lewis acid bronsted acid
Lewis acid bronsted acid Acyl chloride to nitrile
Acyl chloride to nitrile