DES REvolution DES Selection Evidence Impact and Unmet














- Slides: 23

DES (R)Evolution DES Selection: Evidence, Impact and Unmet Needs David E. Kandzari, MD Director, Interventional Cardiology Research Scripps Clinic La Jolla, California kandzari. david@scrippshealth. org

DISCLOSURES David E. Kandzari, MD Consulting Fees – Abbott Vascular, Cordis, a Johnson & Johnson company, Edwards Lifesciences LLC , Medtronic Cardio. Vascular, Inc. , Micell Technologies Grants/Contracted Research – Abbott Vascular, Cordis, a Johnson & Johnson company, Medtronic Cardio. Vascular, Inc. I intend to reference unlabeled/unapproved uses of drugs or devices in my presentation. I intend to reference drug eluting stents for off-label indications.

Disclosure Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below Affiliation/Financial Relationship Company Grant/Research Support Abbott Vascular, Cordis Corporation, Medtronic Cardio. Vascular Consulting Fees/Honoraria Abbott Vascular, Cordis Corporation, Medtronic Cardio. Vascular, Micell Technologies, Terumo Medical Major Stock Shareholder/Equity None Royalty Income None Ownership/Founder None Intellectual Property Rights None Other Financial Benefit None

What Do We Know About DES in 2010? Profound, durable reduction in need for repeat revascularization From RCTs, no overall differences in D/MI/ST, now entering 6 th year of follow-up ‘Off Label’ does not mean ‘Unstudied’ Possibly lower MI and death compared with bare metal stents The story of safety and efficacy with DES does not stop at the primary endpoint Emerging differences in efficacy and safety endpoints between DES, no ‘class effect’

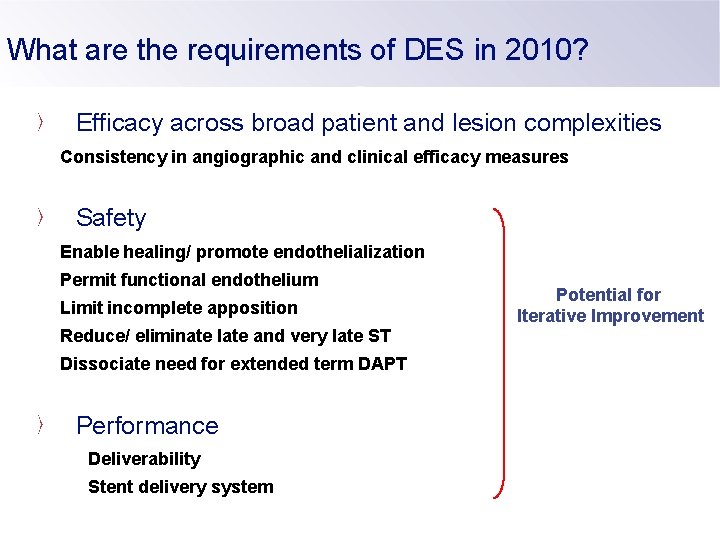
What are the requirements of DES in 2010? Efficacy across broad patient and lesion complexities Consistency in angiographic and clinical efficacy measures Safety Enable healing/ promote endothelialization Permit functional endothelium Limit incomplete apposition Reduce/ eliminate late and very late ST Dissociate need for extended term DAPT Performance Deliverability Stent delivery system Potential for Iterative Improvement

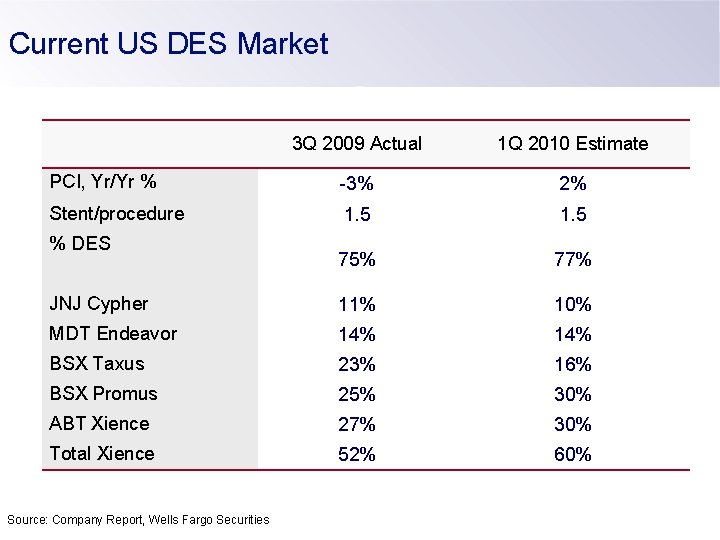
Current US DES Market 3 Q 2009 Actual 1 Q 2010 Estimate PCI, Yr/Yr % -3% 2% Stent/procedure 1. 5 75% 77% JNJ Cypher 11% 10% MDT Endeavor 14% BSX Taxus 23% 16% BSX Promus 25% 30% ABT Xience 27% 30% Total Xience 52% 60% % DES Source: Company Report, Wells Fargo Securities

What are we complaining about? MAUDE: Manufacturer and User Facility Device Experience Search Terms: Xience, Promus, Endeavor Failure to deliver Edge dissection Perforation Stent thrombosis Dislodged stent/ retention Restenosis Fracture www. accessdata. fda. gov/scripts/cdrh/cfdocs/cf. MAUDE, accessed Feb 4, 2010

Impact of Clinical Trials Often Exceeds Conclusions from the Study Results Alone • Sheer number of clinical trials • Departure from evidence-based to media based medicine • Industry competitive landscape Market potential of new device approval • Personalities involved Physicians (Non-interventionalists) Professional societies (ACC, SCAI, AHA, ESC) Regulatory agencies (FDA Panel 12/2006, NICE) Political interest groups and congressional oversight committees

Data Fatigue “…I think physicians are just barraged with studies. I think they are almost in a position where we have lost track of what center is. “ —Ray Elliott President and CEO, Boston Scientific January 12, 2010 JPMorgan Healthcare Conference

Clinical Decision Making Based on Clinical Trials Evolution of Comparative DES Trials Trial Primary Endpoint 1° Endpoint Conclusion Comment SIRIUS 2003 9 mo TVF SES superior to BMS TLR TAXUS IV 2004 9 mo MACE PES superior to BMS TLR REALITY 2006 8 mo in-lesion ABR SES, PES equivalent ABR 1 y MACE: P=NS for SES vs PES SIRTAX 2005 9 mo MACE SES superior to PES TLR ZEST 2009 1 y MACE SES, ZES superior to PES ENDEAVOR IV D/MI: P=NS for all DES TLR: SES< ZES < PES ZES 3 yrs: Cardiac death/MI, VLST 9 mo TVF ZES noninferior to PES SPIRIT IV 2009 1 y TLF EES superior to PES TV-MI, TLR COMPARE 2009 1 y All death, MI, TVR EES superior to PES MI, TVR 2008 Red highlights significant difference and driver of composite endpoint. ZES 3 yrs DM: Cardiac death/MI

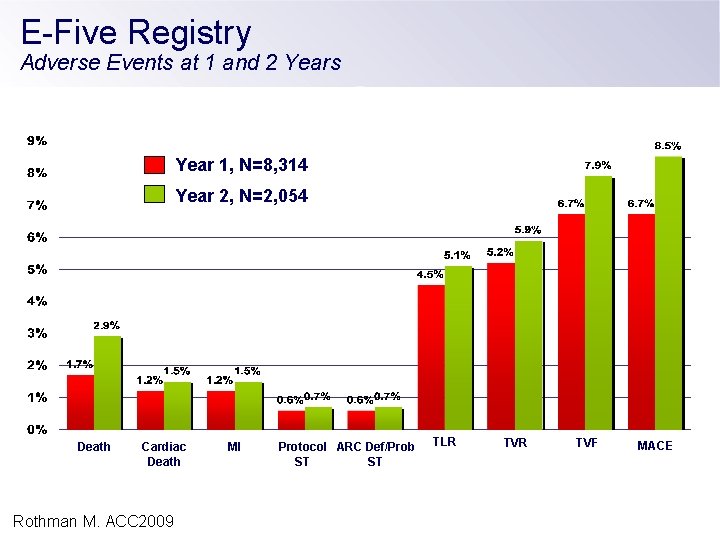
E-Five Registry Adverse Events at 1 and 2 Years Year 1, N=8, 314 Year 2, N=2, 054 Death Cardiac Death Rothman M. ACC 2009 MI Protocol ARC Def/Prob ST ST TLR TVF MACE

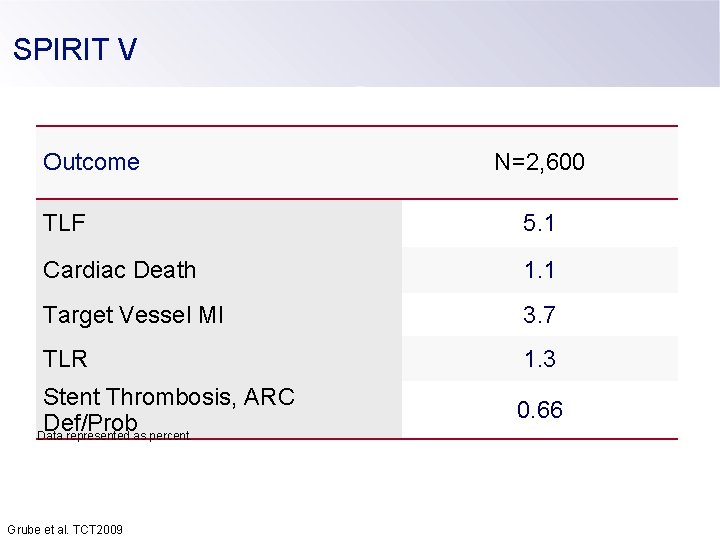
SPIRIT V Outcome N=2, 600 TLF 5. 1 Cardiac Death 1. 1 Target Vessel MI 3. 7 TLR 1. 3 Stent Thrombosis, ARC Def/Prob Data represented as percent. 0. 66 Grube et al. TCT 2009

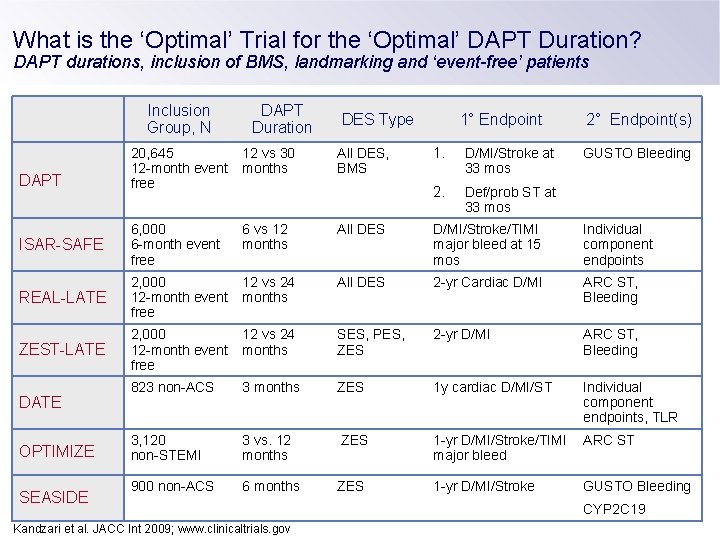
What is the ‘Optimal’ Trial for the ‘Optimal’ DAPT Duration? DAPT durations, inclusion of BMS, landmarking and ‘event-free’ patients Inclusion Group, N DAPT Duration DES Type 1. D/MI/Stroke at 33 mos 2. Def/prob ST at 33 mos 2° Endpoint(s) 20, 645 12 -month event free 12 vs 30 months ISAR-SAFE 6, 000 6 -month event free 6 vs 12 months All DES D/MI/Stroke/TIMI major bleed at 15 mos Individual component endpoints REAL-LATE 2, 000 12 -month event free 12 vs 24 months All DES 2 -yr Cardiac D/MI ARC ST, Bleeding ZEST-LATE 2, 000 12 -month event free 12 vs 24 months SES, PES, ZES 2 -yr D/MI ARC ST, Bleeding 823 non-ACS 3 months ZES 1 y cardiac D/MI/ST Individual component endpoints, TLR 3, 120 non-STEMI 3 vs. 12 months ZES 1 -yr D/MI/Stroke/TIMI major bleed ARC ST 900 non-ACS 6 months ZES 1 -yr D/MI/Stroke GUSTO Bleeding DAPT DATE OPTIMIZE SEASIDE Kandzari et al. JACC Int 2009; www. clinicaltrials. gov All DES, BMS 1° Endpoint GUSTO Bleeding CYP 2 C 19

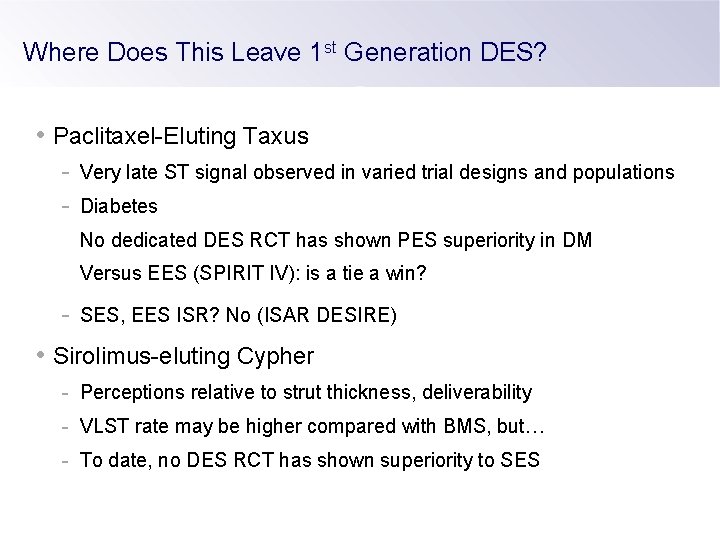
Where Does This Leave 1 st Generation DES? • Paclitaxel-Eluting Taxus - Very late ST signal observed in varied trial designs and populations - Diabetes No dedicated DES RCT has shown PES superiority in DM Versus EES (SPIRIT IV): is a tie a win? - SES, EES ISR? No (ISAR DESIRE) • Sirolimus-eluting Cypher - Perceptions relative to strut thickness, deliverability - VLST rate may be higher compared with BMS, but… - To date, no DES RCT has shown superiority to SES

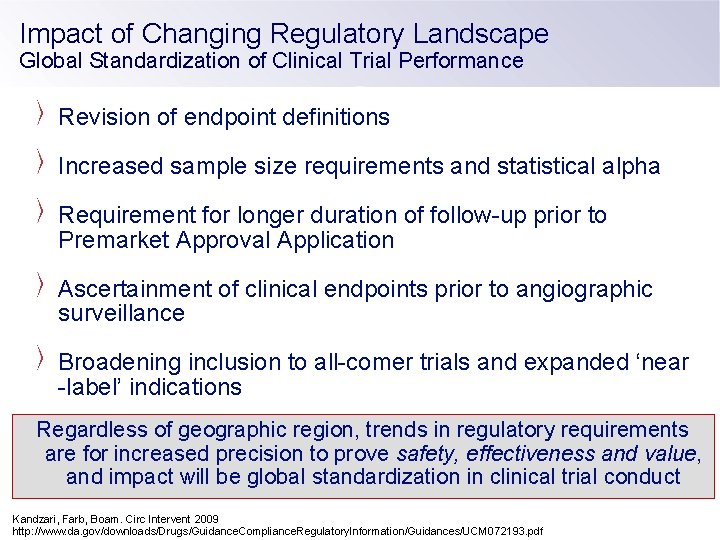
Impact of Changing Regulatory Landscape Global Standardization of Clinical Trial Performance Revision of endpoint definitions Increased sample size requirements and statistical alpha Requirement for longer duration of follow-up prior to Premarket Approval Application Ascertainment of clinical endpoints prior to angiographic surveillance Broadening inclusion to all-comer trials and expanded ‘near -label’ indications Regardless of geographic region, trends in regulatory requirements are for increased precision to prove safety, effectiveness and value, and impact will be global standardization in clinical trial conduct Kandzari, Farb, Boam. Circ Intervent 2009 http: //www. da. gov/downloads/Drugs/Guidance. Compliance. Regulatory. Information/Guidances/UCM 072193. pdf

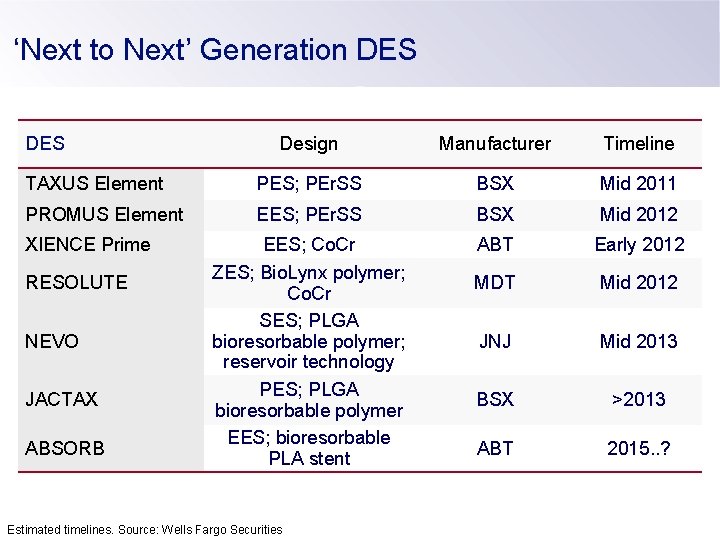
‘Next to Next’ Generation DES Design Manufacturer Timeline TAXUS Element PES; PEr. SS BSX Mid 2011 PROMUS Element EES; PEr. SS BSX Mid 2012 EES; Co. Cr ZES; Bio. Lynx polymer; Co. Cr SES; PLGA bioresorbable polymer; reservoir technology PES; PLGA bioresorbable polymer EES; bioresorbable PLA stent ABT Early 2012 MDT Mid 2012 JNJ Mid 2013 BSX >2013 ABT 2015. . ? XIENCE Prime RESOLUTE NEVO JACTAX ABSORB Estimated timelines. Source: Wells Fargo Securities

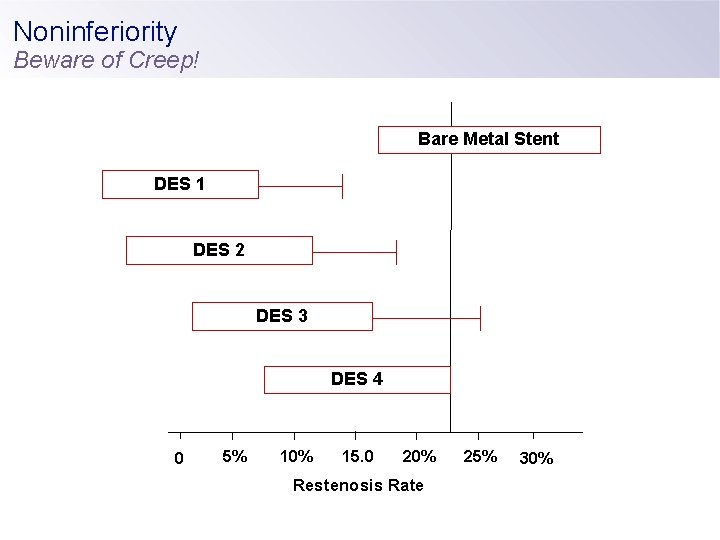
Noninferiority Beware of Creep! Bare Metal Stent DES 1 DES 2 DES 3 DES 4 0 5% 10% 15. 0 20% Restenosis Rate 25% 30%

When P<0. 05 is no longer ‘significant’ Why should(n’t) differences exist between DES? • Below a certain threshold (eg, late loss), all DES are clinically equivalent in efficacy and safety • Convenience - Facilitate clinical trials performance - Justification for perceptions of device performance, eg, deliverability - Standardize regulatory approval pathways


When P<0. 05 is no longer ‘significant’ Why should(n’t) differences exist between DES? • Below a certain threshold (eg, late loss), all DES are clinically equivalent in efficacy and safety • Convenience - Facilitate clinical trials performance - Justification for perceptions of device performance, eg, deliverability - Standardize regulatory approval pathways • Cost • “Indirects” (consumer/sales relationship) • “I don’t see this in my practice…. ”

From Clinical Trials to Clinical Practice 38 RCTs 18, 000 pts “A p-value is no substitute for a brain. ” — Anonymous Stone, Pocock. JACC 2010

Thematic Approach to Comparative Trials DES Example Is there a consistency in efficacy for a DES program? Consistency in safety? [With some exceptions] are the results in order with clinical trials of similar design? What are our expectations for a new generation DES? - Is ‘as good as’ good enough? - Must a ‘new, but similar’ DES demonstrate similar head-to- head outcomes or is inference good enough?

Iterative Development of DES What Does ‘Next Generation’ Really Mean? Until recently, the history and psychology of interventional cardiology has been consumed by anticipation of ‘what’s next’ and that the assumption of ‘what’s next’ is better Adoption of ‘evidence based medicine’ does not occur by intent alone, and ultimately there are many factors independent of trials that influence clinical decision making The responsibility for health care practitioners to understand trial design, methods and pitfalls has never been greater As newer DES are introduced, clinical adoption will be driven more by intuition than scientific evidence as the opportunity to refine outcomes is increasingly difficult There are (and were) many reasons to dismiss the notion that differences may exist between DES, yet EES and ZES have exposed this reality For now, clinical development of new technologies should not simply focus on device approval but also inform current clinical practice Our ‘future’ relates more to forthcoming trials focused more on clinical strategy rather than novel device technologies
 Unmet financial need
Unmet financial need Unmet needs in severe asthma
Unmet needs in severe asthma How did charles dickens impact the industrial revolution
How did charles dickens impact the industrial revolution Difference between testimonial and physical evidence
Difference between testimonial and physical evidence Des des des
Des des des Example of a warrant in writing
Example of a warrant in writing Two way selection and multiway selection
Two way selection and multiway selection Multiway selection in c
Multiway selection in c Mass selection and pure line selection
Mass selection and pure line selection Russian revolution vs french revolution
Russian revolution vs french revolution How could the french revolution have been avoided
How could the french revolution have been avoided Definition of third agricultural revolution
Definition of third agricultural revolution Secondary sources
Secondary sources Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Secondary sources
Secondary sources Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Why is fiber considered class evidence
Why is fiber considered class evidence Class evidence vs individual evidence
Class evidence vs individual evidence Class evidence vs individual evidence
Class evidence vs individual evidence Ecologic fallacy
Ecologic fallacy Balancing selection vs stabilizing selection
Balancing selection vs stabilizing selection Artificial selection vs natural selection
Artificial selection vs natural selection K selection r selection
K selection r selection