COVID19 Vaccine Update Wafaa ElSadr MD MPH MPA




- Slides: 16

COVID-19 Vaccine Update Wafaa El-Sadr, MD, MPH, MPA ICAP at Columbia University January 19, 2021

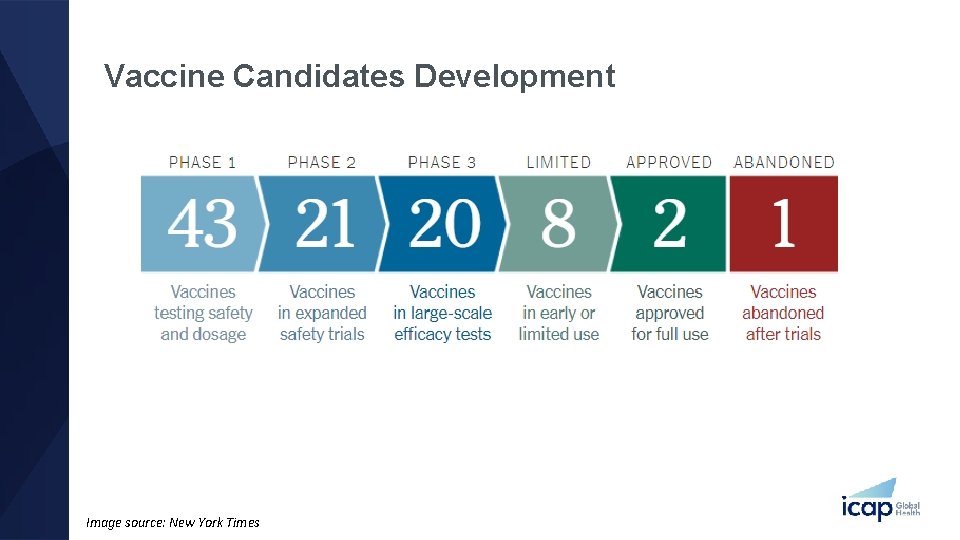
Vaccine Candidates Development Image source: New York Times

How were vaccines developed so quickly? • Vaccine developers had a head start • Years of advanced research had been conducted on related coronaviruses that cause SARS (evere acute respiratory syndrome) and MERS (Middle East respiratory syndrome) • No short-cuts were taken • No steps in the typical vaccine development process were skipped. Rather, they occurred in parallel. • Manufacturing/ infrastructure development occurred simultaneously • Factories for manufacturing, distribution centers, etc. were built as the trials were happening • Enormous interest in COVID-19 vaccines motivate rapid enrollment • Existing processes for emergency use authorization (EUA) were utilized • The FDA EUA process requires decisions to be made according to science and data that has been carefully reviewed by independent bodies

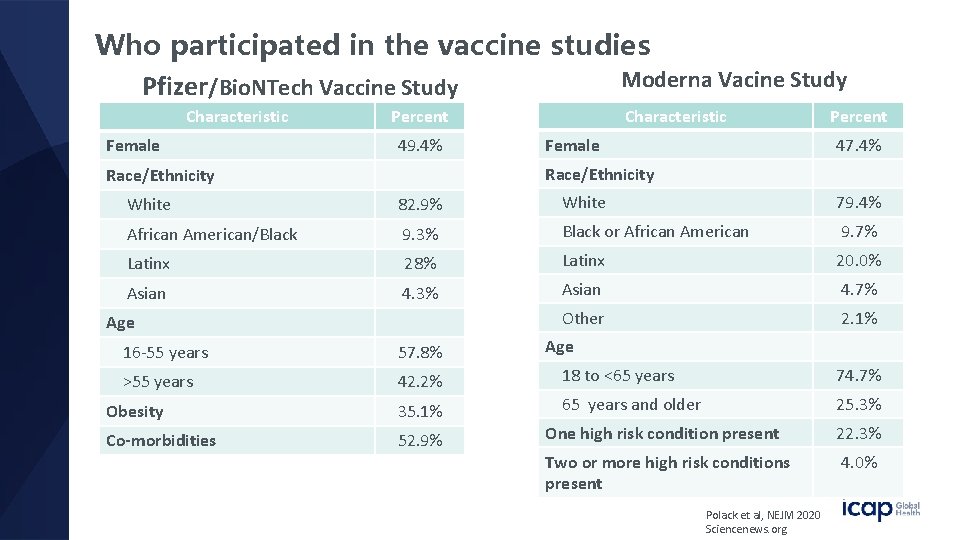
Who participated in the vaccine studies Moderna Vacine Study Pfizer/Bio. NTech Vaccine Study Characteristic Female Percent 49. 4% Characteristic Female Percent 47. 4% Race/Ethnicity White 82. 9% White 79. 4% African American/Black 9. 3% Black or African American 9. 7% Latinx 28% Latinx 20. 0% Asian 4. 3% Asian 4. 7% Other 2. 1% Age 16 -55 years 57. 8% >55 years 42. 2% 18 to <65 years 74. 7% Obesity 35. 1% 65 years and older 25. 3% Co-morbidities 52. 9% One high risk condition present 22. 3% Two or more high risk conditions present 4. 0% Polack et al, NEJM 2020 Sciencenews. org

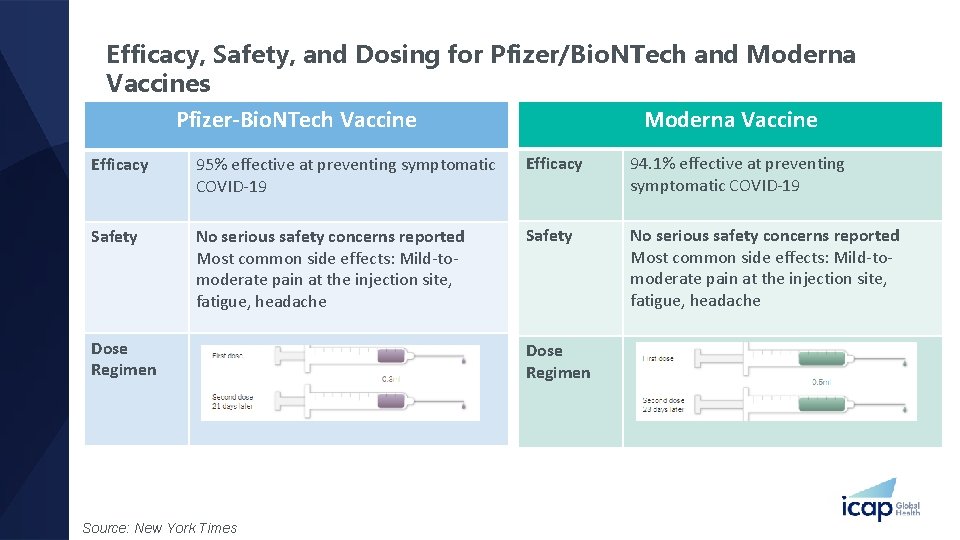
Efficacy, Safety, and Dosing for Pfizer/Bio. NTech and Moderna Vaccines Moderna Vaccine Pfizer-Bio. NTech Vaccine Efficacy 95% effective at preventing symptomatic COVID-19 Efficacy 94. 1% effective at preventing symptomatic COVID-19 Safety No serious safety concerns reported Most common side effects: Mild-tomoderate pain at the injection site, fatigue, headache Dose Regimen Source: New York Times Dose Regimen

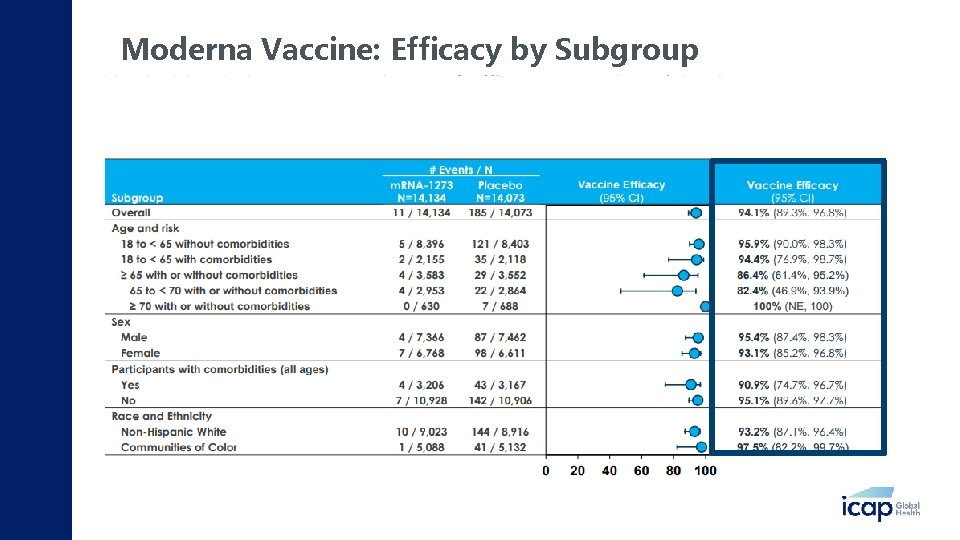
Moderna Vaccine: Efficacy by Subgroup

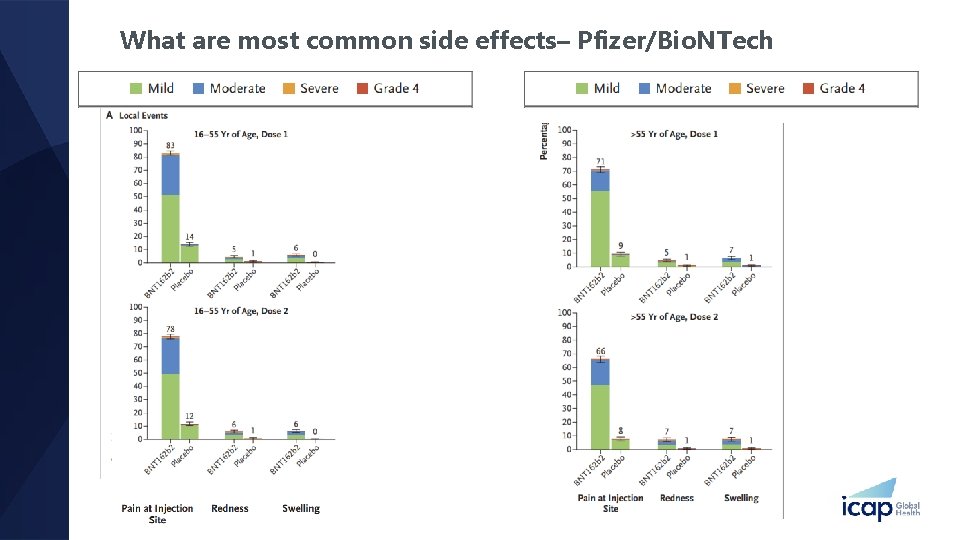
What are most common side effects– Pfizer/Bio. NTech

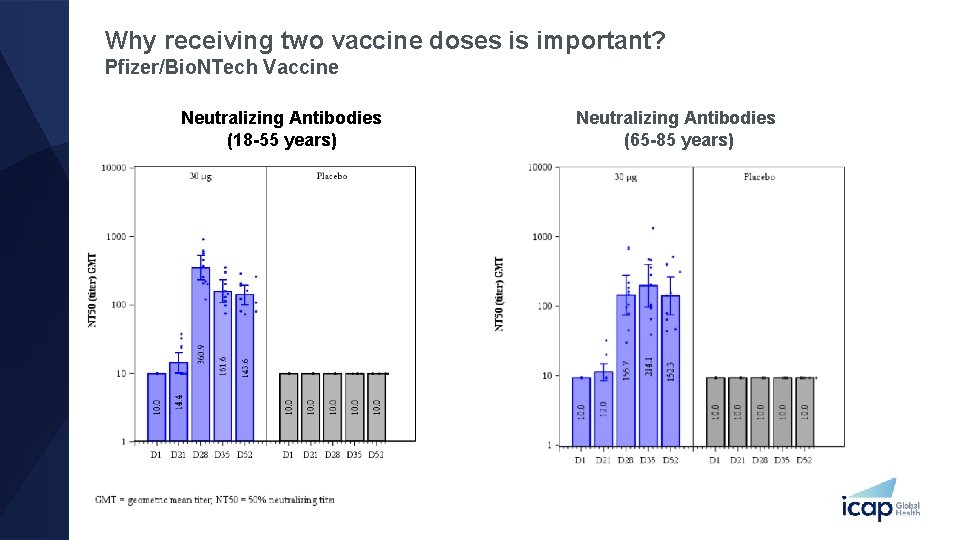
Why receiving two vaccine doses is important? Pfizer/Bio. NTech Vaccine Neutralizing Antibodies (18 -55 years) Neutralizing Antibodies (65 -85 years)

Vaccine Safety Monitoring (US) • Active surveillance: Side effects reported by vaccine recipients: • Passive surveillance: Side effects reported by healthcare professionals, vaccine manufacturers, and the public: Source: CDC

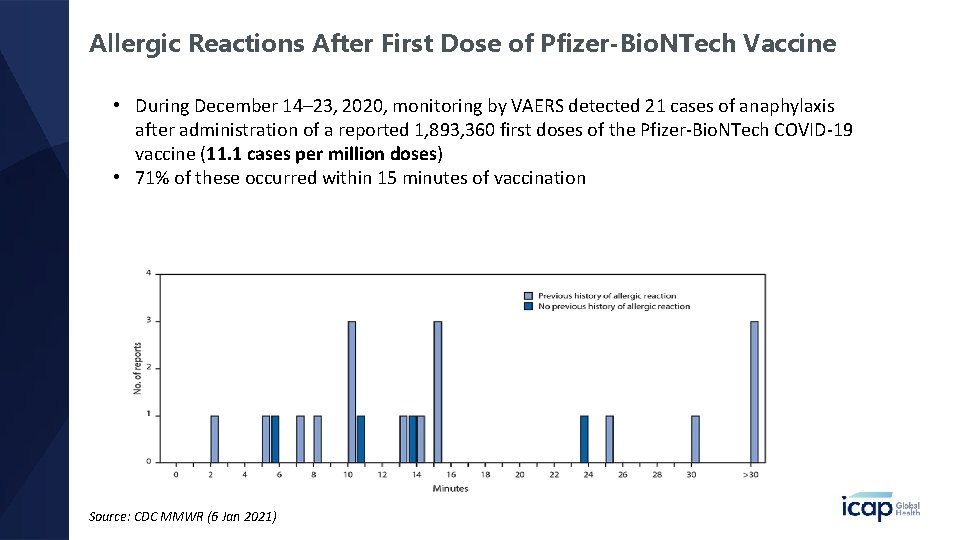
Allergic Reactions After First Dose of Pfizer-Bio. NTech Vaccine • During December 14– 23, 2020, monitoring by VAERS detected 21 cases of anaphylaxis after administration of a reported 1, 893, 360 first doses of the Pfizer-Bio. NTech COVID-19 vaccine (11. 1 cases per million doses) • 71% of these occurred within 15 minutes of vaccination Source: CDC MMWR (6 Jan 2021)

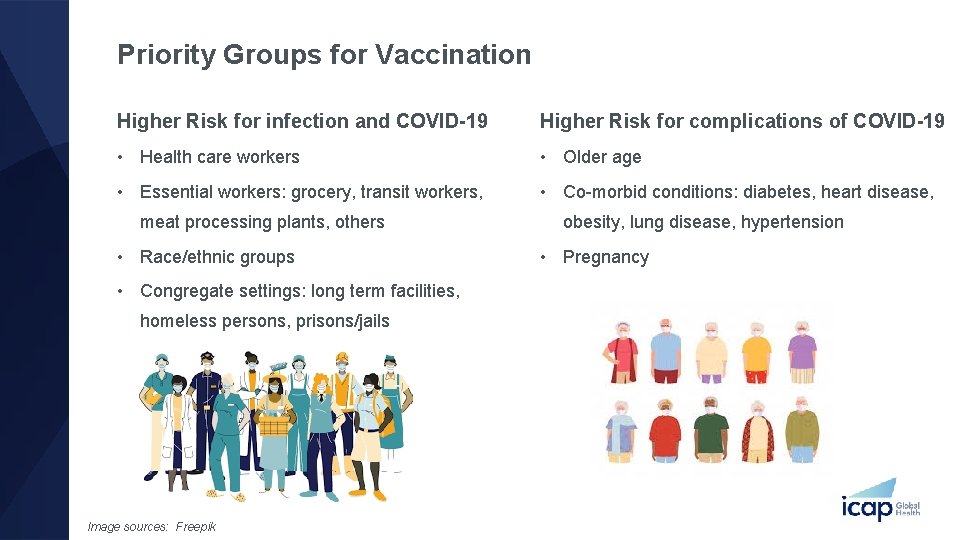
Priority Groups for Vaccination Higher Risk for infection and COVID-19 Higher Risk for complications of COVID-19 • Health care workers • Older age • Essential workers: grocery, transit workers, • Co-morbid conditions: diabetes, heart disease, meat processing plants, others • Race/ethnic groups • Congregate settings: long term facilities, homeless persons, prisons/jails Image sources: Freepik obesity, lung disease, hypertension • Pregnancy

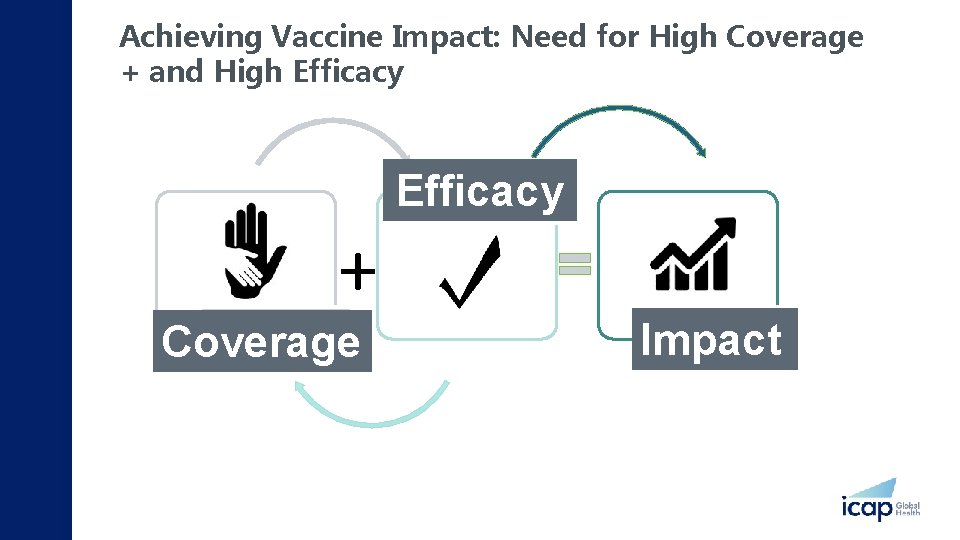
Achieving Vaccine Impact: Need for High Coverage + and High Efficacy Quality Efficacy + Utilization Coverage Health Impact Adapted Margaret Kruk 2013

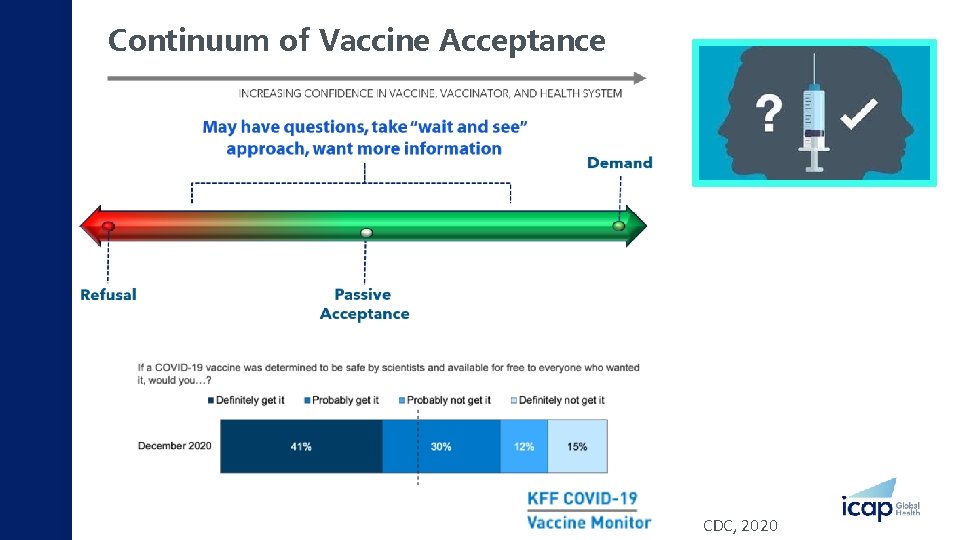
Continuum of Vaccine Acceptance CDC, 2020

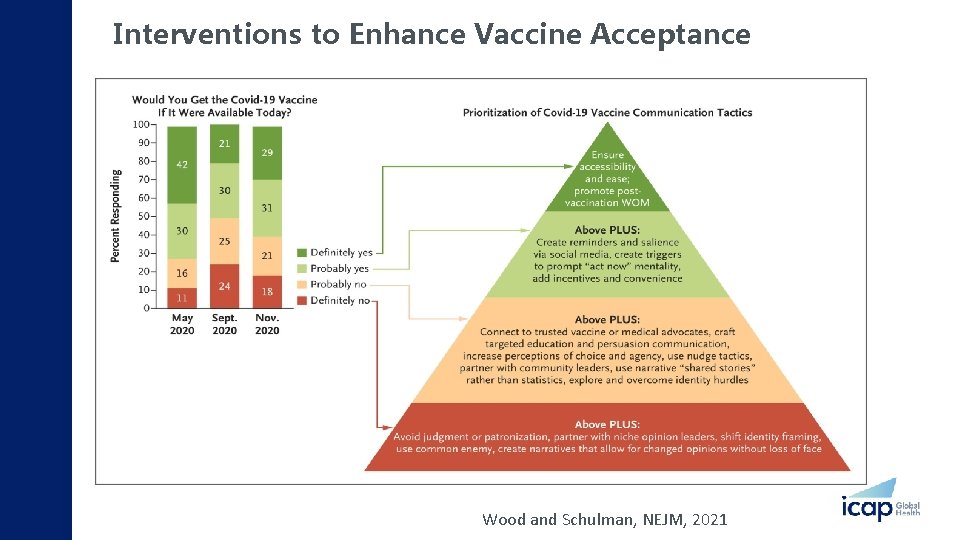
Interventions to Enhance Vaccine Acceptance Wood and Schulman, NEJM, 2021

Unanswered Questions/Ongoing Research • Prevention of SARS-Co. V-2 infection • Durability of protection • Efficacy after 1 dose • Safety for children • Safety for pregnant and breastfeeding persons • Efficacy and safety in immunosuppressed individuals • Efficacy and safety of combining different types of vaccines

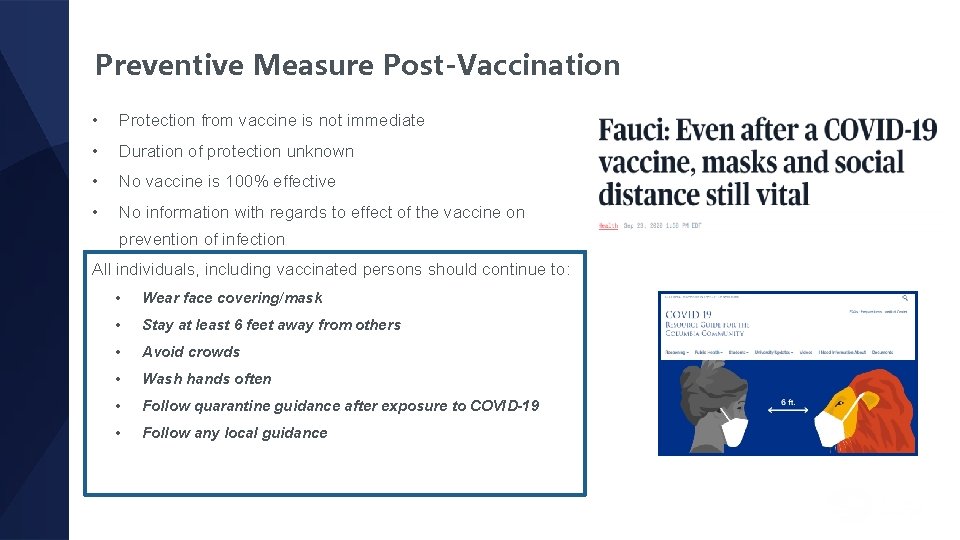
Preventive Measure Post-Vaccination • Protection from vaccine is not immediate • Duration of protection unknown • No vaccine is 100% effective • No information with regards to effect of the vaccine on prevention of infection All individuals, including vaccinated persons should continue to: • Wear face covering/mask • Stay at least 6 feet away from others • Avoid crowds • Wash hands often • Follow quarantine guidance after exposure to COVID-19 • Follow any local guidance
 How dr. wafaa elsadr epidemiology professor
How dr. wafaa elsadr epidemiology professor How dr. wafaa elsadr epidemiology professor
How dr. wafaa elsadr epidemiology professor Is an alternative of log based recovery.
Is an alternative of log based recovery. Wafaa pronunciation
Wafaa pronunciation Wafaa abdallah
Wafaa abdallah Do if you covid19
Do if you covid19 Covid19 athome rapid what know
Covid19 athome rapid what know What do if test positive covid19
What do if test positive covid19 Http//apps.tujuhbukit.com/covid19
Http//apps.tujuhbukit.com/covid19 Vaksin covid19
Vaksin covid19 Mpa
Mpa Byu student alumni
Byu student alumni Ucf public administration masters
Ucf public administration masters Project server 2003
Project server 2003 Mpa
Mpa Allianz cern mpa
Allianz cern mpa Beton fck
Beton fck