Communication and Optimal Resolution CANDOR Toolkit Module 4











- Slides: 18

Communication and Optimal Resolution (CANDOR) Toolkit Module 4: Event Reporting, Event Investigation and Analysis

Objectives • Define the key elements of a timely and comprehensive event reporting system. • Define the process of a timely and efficient event investigation. • Identify the key components of an effective event analysis. Module 4 2

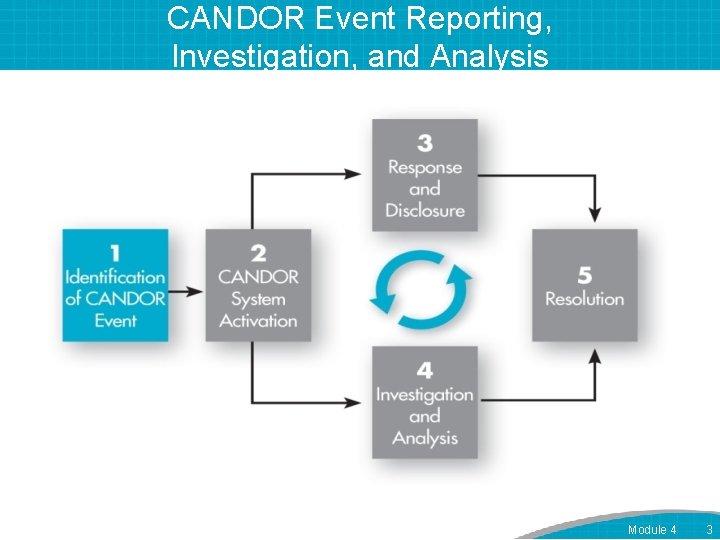
CANDOR Event Reporting, Investigation, and Analysis Module 4 3

1 Event Reporting Culture “The single greatest impediment to error prevention in the medical industry is that we punish people for making mistakes. ” Dr. Lucian Leape Professor, Harvard School of Public Health Testimony before Congress on Health Care Quality Improvement

Key Elements of an Event Reporting System Supports a rapid response to harm events. Obtain staff and providers’ feedback post-event. Obtain patient and family feedback post-event. Allows for immediate, anonymous, and/or confidential reporting and input from frontline staff and providers. • Provides potential protection of event analysis from discovery. • Provides immediate and ongoing feedback to reporters. • Protects patients from future harm events. • • Module 4 5

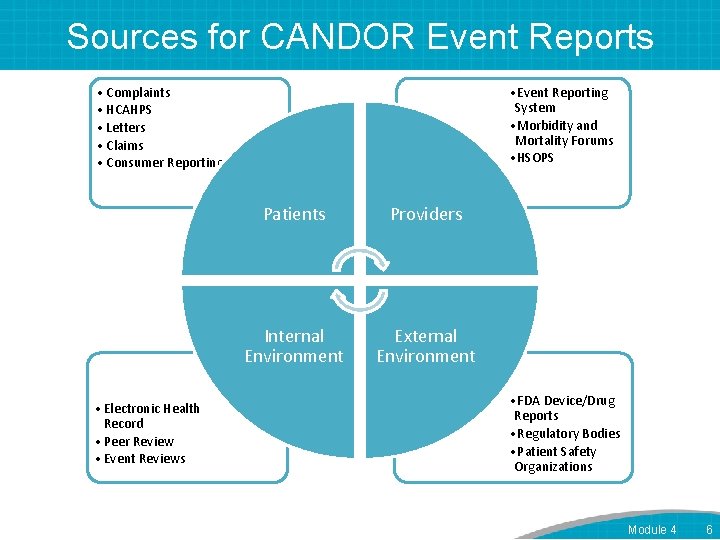
Sources for CANDOR Event Reports • Complaints • HCAHPS • Letters • Claims • Consumer Reporting • Electronic Health Record • Peer Review • Event Reviews • Event Reporting System • Morbidity and Mortality Forums • HSOPS Patients Providers Internal Environment External Environment • FDA Device/Drug Reports • Regulatory Bodies • Patient Safety Organizations Module 4 6

CANDOR Event Checklist Module 4 7

Preparing Staff for a CANDOR Event Reporting System • Ensure staff understands how to prepare, and has the ability to submit, an event report. • Provide guidance on how to report an event. • Conduct training for all staff. • Provide a mechanism for followup and feedback on the event. • Support managers. • Seek feedback to identify ways to make the process “user friendly. ” Module 4 8

Event Reporting Outcomes • • • Caregiver support Patient and family engagement and support Continuous organizational learning Innovative solutions Improved culture of safety Module 4 9

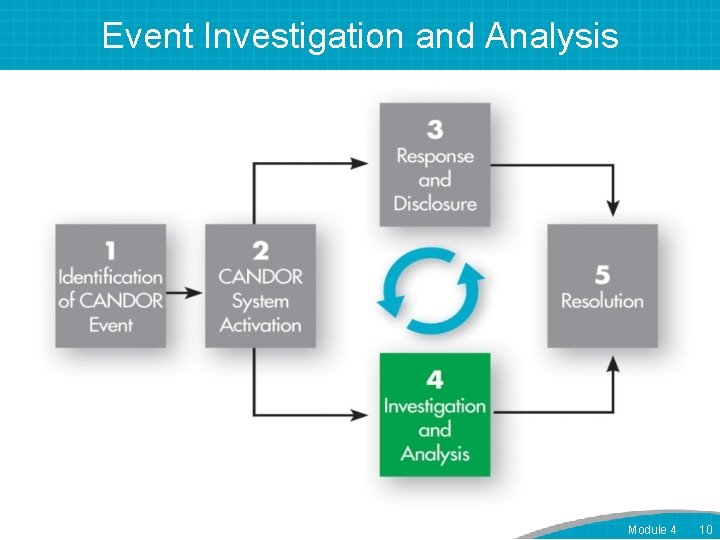
Event Investigation and Analysis Module 4 10

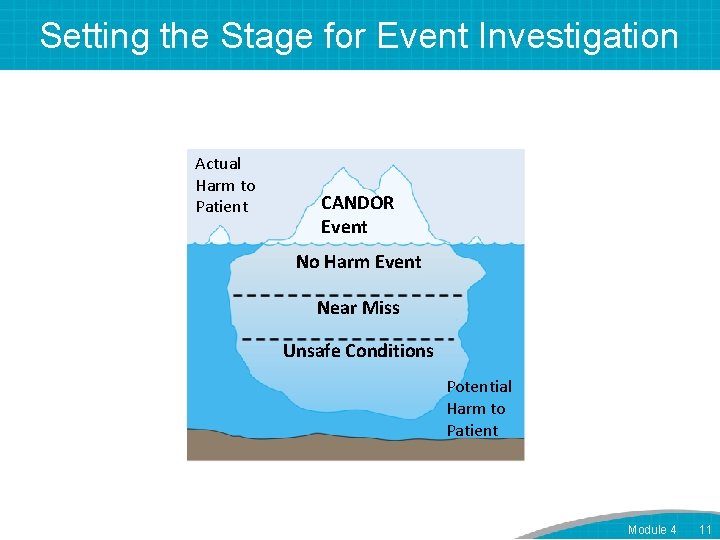
Setting the Stage for Event Investigation Actual Harm to Patient CANDOR Event No Harm Event Near Miss Unsafe Conditions Potential Harm to Patient Module 4 11

System And Individual Accountability 2 • Why did the event happen? How prevalent are the behaviors associated with the event? • System accountability – Processes, policies – Prevention mechanisms • Individual accountability – At-risk behaviors – Performance factors Additional Resource: AHRQ CUSP Toolkit - Apply CUSP Module 4 12

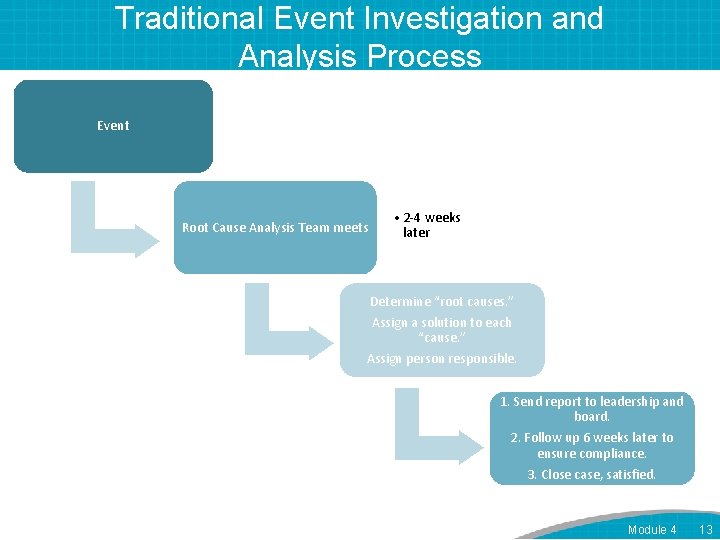
Traditional Event Investigation and Analysis Process Event Root Cause Analysis Team meets • 2 -4 weeks later Determine “root causes. ” Assign a solution to each “cause. ” Assign person responsible. 1. Send report to leadership and board. 2. Follow up 6 weeks later to ensure compliance. 3. Close case, satisfied. Module 4 13

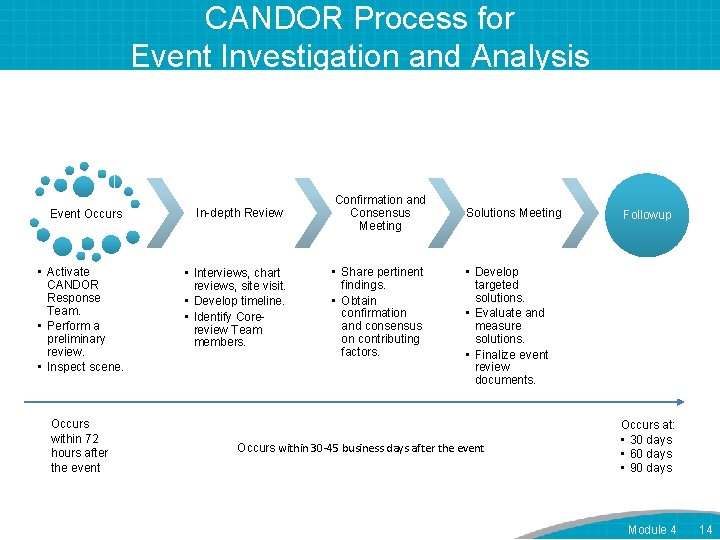
CANDOR Process for Event Investigation and Analysis Event Occurs • Activate CANDOR Response Team. • Perform a preliminary review. • Inspect scene. Occurs within 72 hours after the event In-depth Review • Interviews, chart reviews, site visit. • Develop timeline. • Identify Corereview Team members. Confirmation and Consensus Meeting • Share pertinent findings. • Obtain confirmation and consensus on contributing factors. Solutions Meeting Followup • Develop targeted solutions. • Evaluate and measure solutions. • Finalize event review documents. Occurs within 30 -45 business days after the event Occurs at: • 30 days • 60 days • 90 days Module 4 14

System-Focused Event Investigation and Analysis Guide 3 Module 4 15

Followup • • Patient and family 4, 5 Caregivers Patient and family advisory councils Medical liability carriers – Mello et al: Communication-and-resolution programs: the challenges and lessons learned from six early adopters 6 – Boothman and Hoyler: The University of Michigan's early disclosure and offer program 7 Module 4 16

• • • Event Investigation and Analysis Outcomes Caregiver support Patient and family engagement and support Continuous organizational learning Innovative creation of solutions Impact on the safety culture Module 4 17

References 1. 2. 3. 4. 5. 6. 7. Leape LL. Testimony, United States Congress, House Committee on Veterans’ Affairs, October 12, 1997. Apply CUSP module, CUSP Toolkit. Rockville, MD: Agency for Healthcare Research and Quality. http: //www. ahrq. gov/professionals/education/curriculumtools/cusptoolkit/modules/apply/index. html. Accessed August 8, 2015. Guide to Patient and Family Engagement in Hospital Quality and Safety. Rockville, MD. Agency for Healthcare Research and Quality. http: //www. ahrq. gov/professionals/systems/hospital/engagingfamilies. Accessed July 21, 2015. Carman KL , et al. A Roadmap for Patient and Family Engagement in Healthcare Practice and Research. Gordon and Betty Moore Foundation. Palo Alto, CA; September 2014. http: //patientfamilyengagement. org/#sthash. HZNsl. ZP 1. Wp. Gsc 2 si. dpuf. Accessed July 21, 2015. Parker SH, Krevat SA, Morales CL, Fairbanks RJ. System-Focused Event Investigation and Analysis Guide. Columbia, MD: Med. Star Health. Washington DC: Georgetown University. Mello MM, et al. Communication-and-resolution programs: the challenges and lessons learned from six early adopters. Health Affairs. 33. 1 (2014): 20 -29. http: //content. healthaffairs. org/content/33/1/20. full. pdf+html. Accessed September 10, 2015 Boothman, R and Hoyler MM. The University of Michigan's early disclosure and offer program. Bulletin of the American College of Surgeons. 98. 3 (2013): 21 -25. http: //bulletin. facs. org/2013/03/michigans-early-disclosure/. Accessed August 21, 2015. Module 4 18