Colloidal Solution and its applications DR NEETI MISHRA









- Slides: 12

Colloidal Solution and its applications DR NEETI MISHRA CHEMISTRY DEPARTMENT BHAVANS MEHTA MAHAVIDYALAYA BHARWARI, KAUSHAMBI

What are Colloids and Colloidal Solution Greek word Kolla(glue) and oid(like). Grahms Classification of Colloids and Crystalloids on the basis of diffusion through animal membrane. Na. Cl, in water is the example of crystalloids and in benzene is the example of colloids. Classification on the Basis of Molecular Size True Solution Colloidal Solution Suspension

Colloidal Solution=Dispersed Phase + Dispersion Medium Colloidal solution is heterogenous mixture of dispersed phase and dispersion medium. Depending upon the interaction between dispersed phase and dispersion medium it is of two type. Lyophilic : High Stability, Reversible in nature Lyophobic; Less stable, irreversible Depending upon type of colloidal particle it is of two type Molecular Colloids : Example proteins Colloids : Gold sols Associated

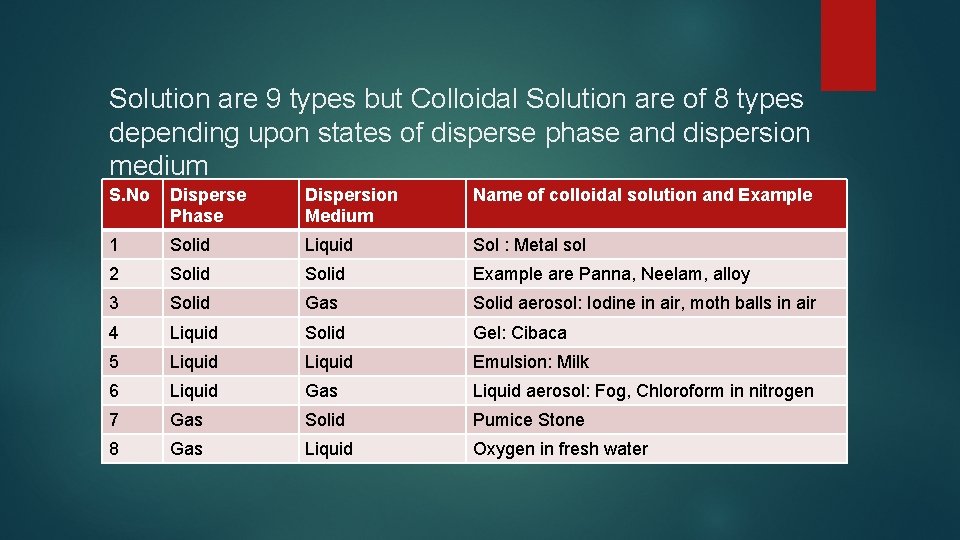
Solution are 9 types but Colloidal Solution are of 8 types depending upon states of disperse phase and dispersion medium S. No Disperse Phase Dispersion Medium Name of colloidal solution and Example 1 Solid Liquid Sol : Metal sol 2 Solid Example are Panna, Neelam, alloy 3 Solid Gas Solid aerosol: Iodine in air, moth balls in air 4 Liquid Solid Gel: Cibaca 5 Liquid Emulsion: Milk 6 Liquid Gas Liquid aerosol: Fog, Chloroform in nitrogen 7 Gas Solid Pumice Stone 8 Gas Liquid Oxygen in fresh water

Characteristics of Colloidal Solution Heterogenous nature visible under ultramicroscope. Solution is turbid. Sometimes color of colloidal solutions depends on size of colloidal particles. Although it is generally argument that Brownian motion and Tyndall effect shown by colloidal solution but it is not so. Lyophilic sol do not show Tyndall effect and Brownian motion. Viscosity and surface tension play different role in colloidal solution

Origin of charge and Stability of Colloidal solution Charge on colloidal particles due to selective adsorption. For example if we take equimolar amount of Ag. NO 3 and Na. I than Ag. I will be precipitated. If Silver nitrate is in excess Then due to selective adsorption of Ag+ on colloidal particle it will acquire positive charge. If Na. I is in excess then due to selective adsorption of I- ion it will acquire negative charge. Due to charge colloidal solution become stable and do come under gravitational pull which leads to coagulation.

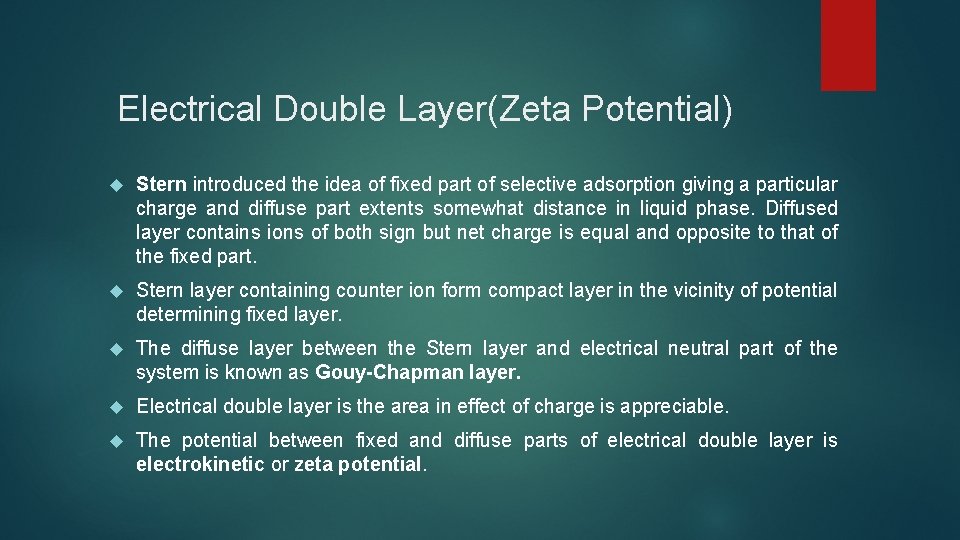
Electrical Double Layer(Zeta Potential) Stern introduced the idea of fixed part of selective adsorption giving a particular charge and diffuse part extents somewhat distance in liquid phase. Diffused layer contains ions of both sign but net charge is equal and opposite to that of the fixed part. Stern layer containing counter ion form compact layer in the vicinity of potential determining fixed layer. The diffuse layer between the Stern layer and electrical neutral part of the system is known as Gouy-Chapman layer. Electrical double layer is the area in effect of charge is appreciable. The potential between fixed and diffuse parts of electrical double layer is electrokinetic or zeta potential.

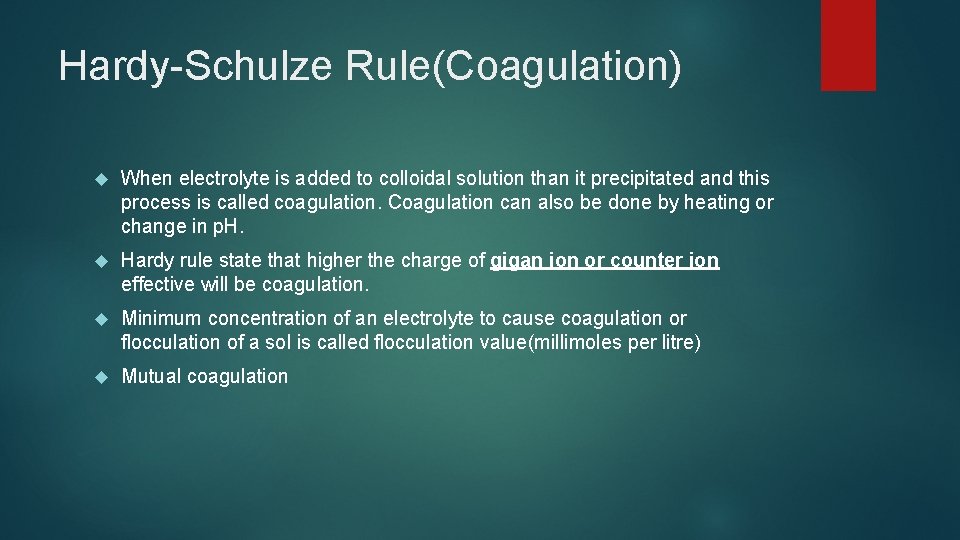
Hardy-Schulze Rule(Coagulation) When electrolyte is added to colloidal solution than it precipitated and this process is called coagulation. Coagulation can also be done by heating or change in p. H. Hardy rule state that higher the charge of gigan ion or counter ion effective will be coagulation. Minimum concentration of an electrolyte to cause coagulation or flocculation of a sol is called flocculation value(millimoles per litre) Mutual coagulation

Purification of colloidal solution Dialysis: Membrane used for the purpose is called dialyser(parchment membrane or animal membrane. Electrodialysis: Used in aquasol. Minimum wastage of water Ultrafiltration: Filter paper impregnated with colloidian and hardened by formaldehyde , the pore size are reduced. Colloidal particle are retained and other impurities passed through dispersion medium. 100% purification leads to coagulation.

Protection and Gold Number Lyophilic sol when added to lyophobic sol than colloidal particle of lyophilic surround the colloidal particle of lyophobic sol and protect it from coagulation when electrolyte is added to it. Therefore lyophilic sol are also called protective colloid. Protective power of lyophilic sol is compare with the help of GOLD NUMBER. Gold number of protective colloid is equal number of milligram of concerned required for coagulation of 10 ml of standard gold sol when 1 ml of 10%Na. Cl solution is added to it. Smaller the gold number higher will be protective power.

Conclusion From this not only food, industry but other application are available Delta formation Drugs given in form of colloidal and artificial kidney machine Artificial rain Sewage disposal problem and solution Uses in metallurgy

Thanks DR NEETI MISHRA