Clinical Outcomes Assessment of the Mitra Clip Percutaneous









- Slides: 14

Clinical Outcomes Assessment of the Mitra. Clip Percutaneous Therapy for High Surgical Risk Patients COAPT Trial Ted Feldman, M. D. , FSCAI FACC FESC Evanston Hospital CRT Cardiovacular Research Technologies Washington, D. C. February 23 rd– 26 th 2013

Ted E. Feldman, MD Consulting: Abbott Laboratories Boston Scientific Corporation Edwards Lifesciences, LLC

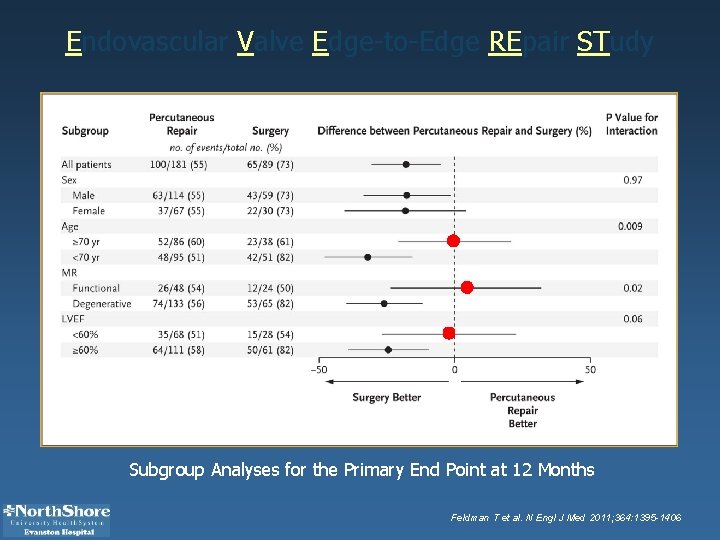
CONCLUSIONS Although percutaneous repair was less effective at reducing mitral regurgitation than conventional surgery, the procedure was associated with superior safety and similar improvements in clinical outcomes.

Endovascular Valve Edge-to-Edge REpair STudy Subgroup Analyses for the Primary End Point at 12 Months Feldman T et al. N Engl J Med 2011; 364: 1395 -1406


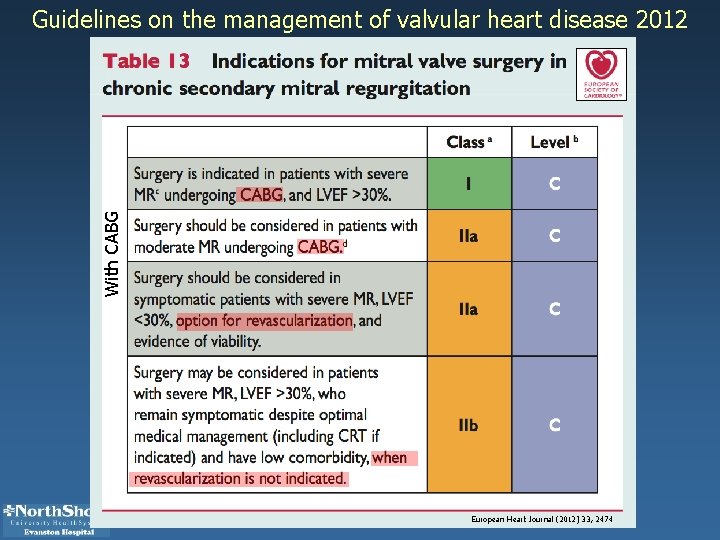
With CABG Guidelines on the management of valvular heart disease 2012 European Heart Journal (2012) 33, 2474

THE COAPT TRIAL Clinical Outcomes Assessment of the Mitra. Clip Percutaneous Therapy for High Surgical Risk

Purpose • evaluate the safety and effectivenessthe Mitra. Clip device in an FMR patient population that is too high risk to undergo mitral valve surgery • study will generate clinical and economic data to support reimbursement and evidence to support the development of treatment guidelines • first RCT to compare non-surgical standard of care treatment to an intervention for MR

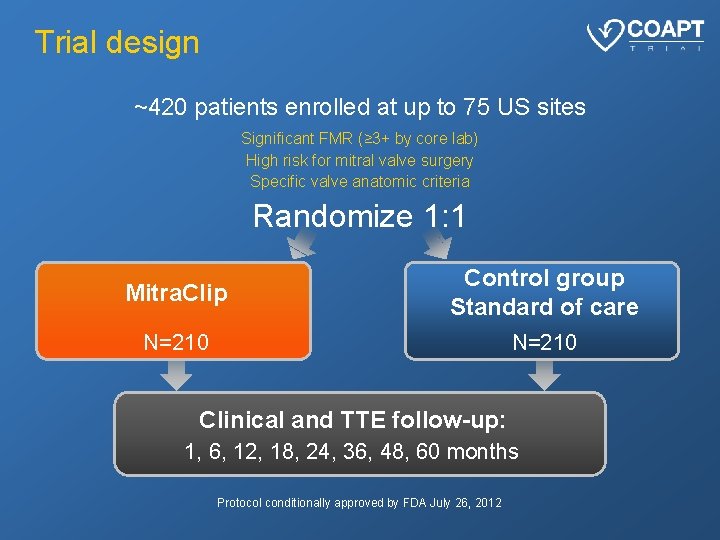
Trial design ~420 patients enrolled at up to 75 US sites Significant FMR (≥ 3+ by core lab) High risk for mitral valve surgery Specific valve anatomic criteria Randomize 1: 1 Mitra. Clip Control group Standard of care N=210 Clinical and TTE follow-up: 1, 6, 12, 18, 24, 36, 48, 60 months Protocol conditionally approved by FDA July 26, 2012

Key Inclusion Criteria • Functional MR ≥ 3+ – ischemic or non-ischemic cardiomyopathy • Symptomatic – NYHA class II, III or ambulatory IV • STS mortality risk is ≥ 8% or Local Site Heart Team concludes that co-morbidities result in a prohibitive predicted operative risk of stroke or death • ≥ 1 HF hospitalization during prior year – and/or BNP ≥ 400 pg/ml – or n. T-pro. BNP ≥ 1600 pg/ml ≤ 90 days • treated per standards for CAD, LV dysfunction, MR or HF including CRT, revascularization, OMT • primary MR jet originates from malcoaptation of A 2 -P 2 scallops

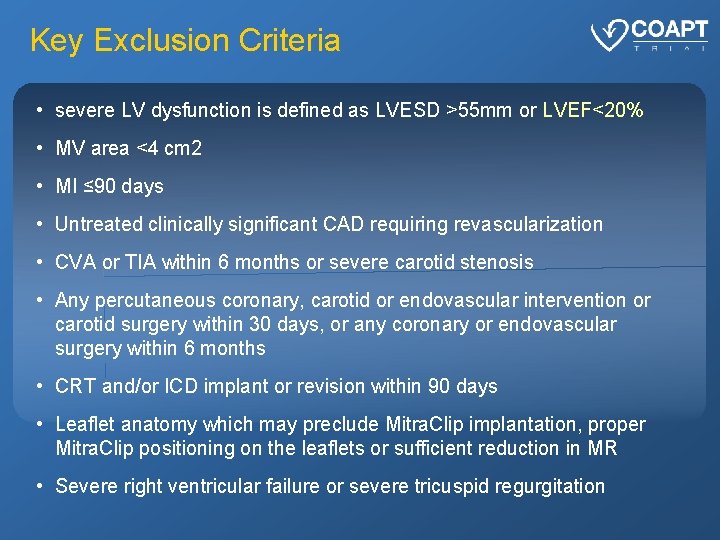
Key Exclusion Criteria • severe LV dysfunction is defined as LVESD >55 mm or LVEF<20% • MV area <4 cm 2 • MI ≤ 90 days • Untreated clinically significant CAD requiring revascularization • CVA or TIA within 6 months or severe carotid stenosis • Any percutaneous coronary, carotid or endovascular intervention or carotid surgery within 30 days, or any coronary or endovascular surgery within 6 months • CRT and/or ICD implant or revision within 90 days • Leaflet anatomy which may preclude Mitra. Clip implantation, proper Mitra. Clip positioning on the leaflets or sufficient reduction in MR • Severe right ventricular failure or severe tricuspid regurgitation

Primary Endpoints • Primary Effectiveness (min 1 -year FU all pts) – Recurrent heart failure hospitalizations • Superiority hypothesis (Andersen-Gill) • Primary Safety (1 year) – Composite of all-cause death, stroke, worsening kidney function, or LVAD or cardiac transplant • Non-inferiority hypothesis

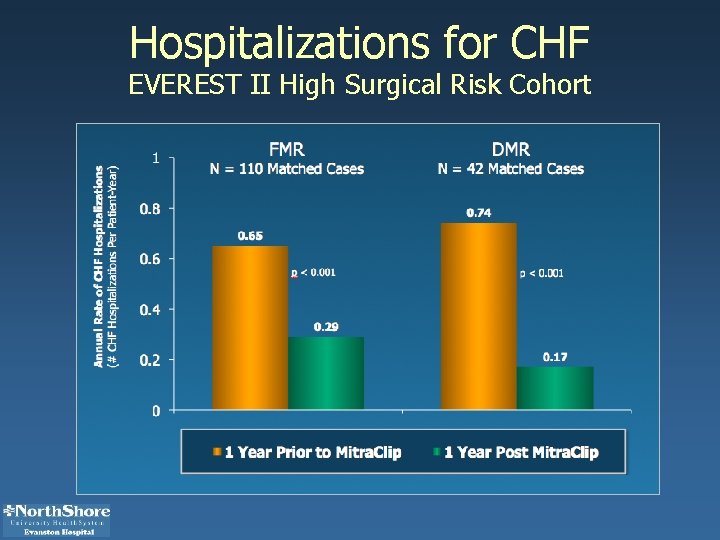
Hospitalizations for CHF EVEREST II High Surgical Risk Cohort

 Dcb clinical outcomes
Dcb clinical outcomes Percutaneous image-guided lumbar decompression (pild)
Percutaneous image-guided lumbar decompression (pild) Percutaneous balloon pericardiotomy
Percutaneous balloon pericardiotomy Percutaneous transhepatic cholangiography
Percutaneous transhepatic cholangiography Amnio vs cvs
Amnio vs cvs Bile color
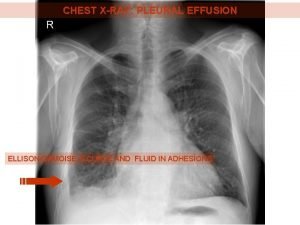
Bile color Chest xray labelled
Chest xray labelled Blackboard outcomes assessment
Blackboard outcomes assessment Clinical comprehensive assessment
Clinical comprehensive assessment Cas clinical assessment
Cas clinical assessment Indirect method of nutritional assessment
Indirect method of nutritional assessment Clinical assessment protocols
Clinical assessment protocols National clinical assessment service
National clinical assessment service Pt among mitra bakti utama
Pt among mitra bakti utama Chiranjib mitra
Chiranjib mitra