Mitra Clip Review of the Data From the




- Slides: 21

Mitra. Clip Review of the Data From the EVEREST Trials Ted Feldman, M. D. , MSCAI FACC FESC Evanston Hospital CRT Cardiovascular Research Technologies Washington D. C. March 4 -6 th, 2018

Ted Feldman MD, MSCAI FACC FESC Disclosure Information The following relationships exist: Grant support: Abbott, BSC, Cardiokinetics, Corvia, Edwards, WL Gore Consultant: Abbott, BSC, Edwards, WL Gore Stock Options: Mitralign Off label use of products and investigational devices will be discussed in this presentation

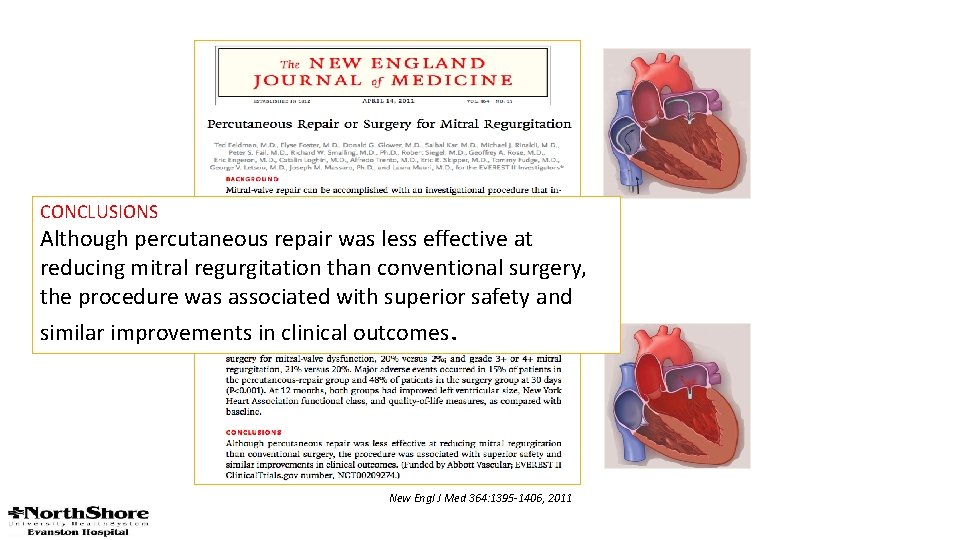
CONCLUSIONS Although percutaneous repair was less effective at reducing mitral regurgitation than conventional surgery, the procedure was associated with superior safety and similar improvements in clinical outcomes. New Engl J Med 364: 1395 -1406, 2011

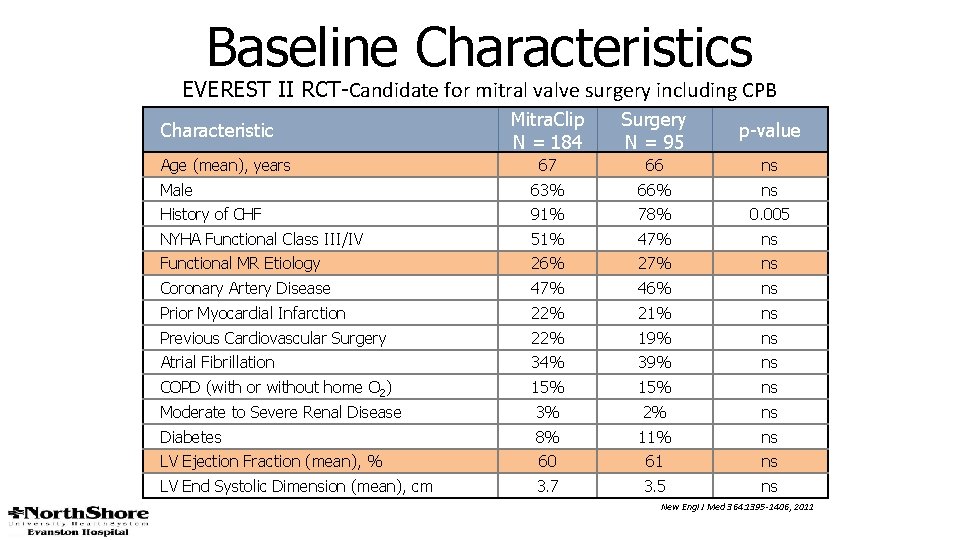
Baseline Characteristics EVEREST II RCT-Candidate for mitral valve surgery including CPB Mitra. Clip N = 184 Surgery N = 95 p-value 67 66 ns Male 63% 66% ns History of CHF 91% 78% 0. 005 NYHA Functional Class III/IV 51% 47% ns Functional MR Etiology 26% 27% ns Coronary Artery Disease 47% 46% ns Prior Myocardial Infarction 22% 21% ns Previous Cardiovascular Surgery 22% 19% ns Atrial Fibrillation 34% 39% ns COPD (with or without home O 2) 15% ns Moderate to Severe Renal Disease 3% 2% ns Diabetes 8% 11% ns LV Ejection Fraction (mean), % 60 61 ns LV End Systolic Dimension (mean), cm 3. 7 3. 5 ns Characteristic Age (mean), years New Engl J Med 364: 1395 -1406, 2011


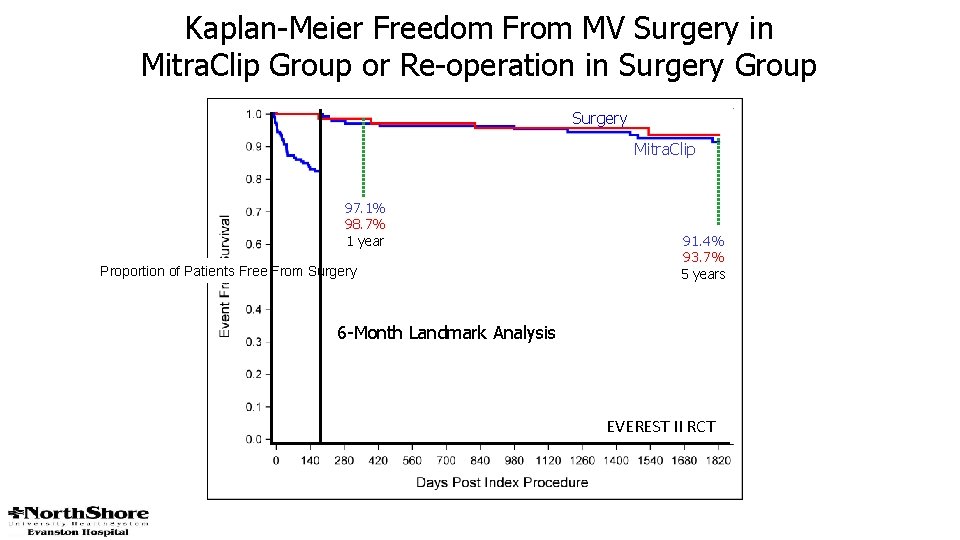
Kaplan-Meier Freedom From MV Surgery in Mitra. Clip Group or Re-operation in Surgery Group Surgery Mitra. Clip 97. 1% 98. 7% 1 year Proportion of Patients Free From Surgery 91. 4% 93. 7% 5 years 6 -Month Landmark Analysis EVEREST II RCT

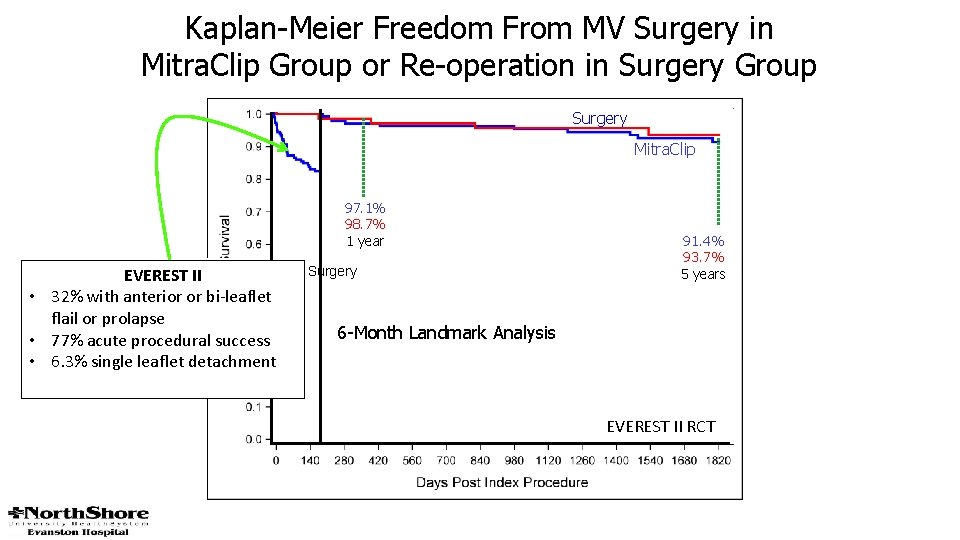
Kaplan-Meier Freedom From MV Surgery in Mitra. Clip Group or Re-operation in Surgery Group Surgery Mitra. Clip 97. 1% 98. 7% 1 year Proportion of Patients Free From Surgery EVEREST II • 32% with anterior or bi-leaflet flail or prolapse • 77% acute procedural success • 6. 3% single leaflet detachment 91. 4% 93. 7% 5 years 6 -Month Landmark Analysis EVEREST II RCT

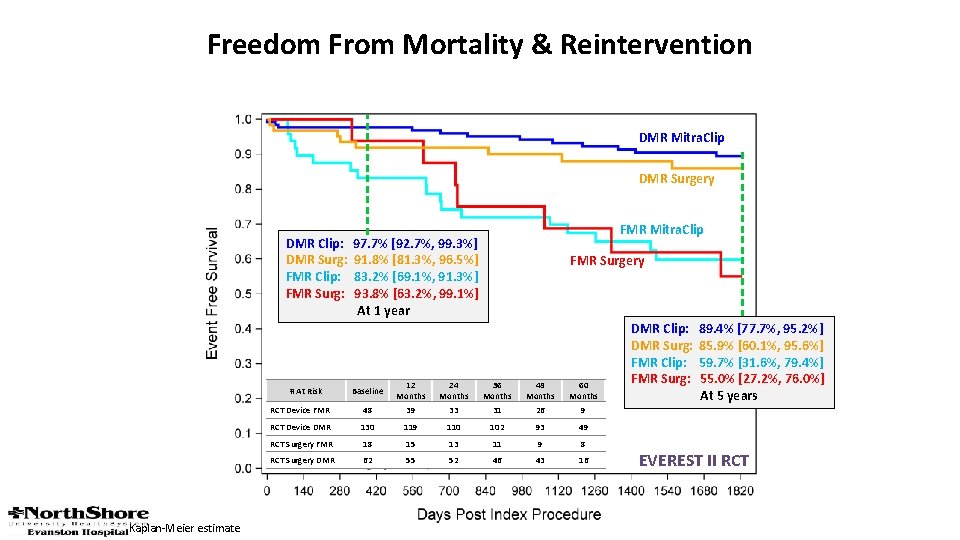
Freedom From Mortality & Reintervention DMR Mitra. Clip DMR Surgery DMR Clip: DMR Surg: FMR Clip: FMR Surg: FMR Surgery Baseline 12 Months 24 Months 36 Months 48 Months 60 Months RCT Device FMR 48 39 33 31 26 9 RCT Device DMR 130 119 110 102 93 49 RCT Surgery FMR 18 15 13 11 9 8 RCT Surgery DMR 62 55 52 46 43 16 # At Risk Kaplan-Meier estimate FMR Mitra. Clip 97. 7% [92. 7%, 99. 3%] 91. 8% [81. 3%, 96. 5%] 83. 2% [69. 1%, 91. 3%] 93. 8% [63. 2%, 99. 1%] At 1 year DMR Clip: DMR Surg: FMR Clip: FMR Surg: 89. 4% [77. 7%, 95. 2%] 85. 9% [60. 1%, 95. 6%] 59. 7% [31. 6%, 79. 4%] 55. 0% [27. 2%, 76. 0%] At 5 years EVEREST II RCT

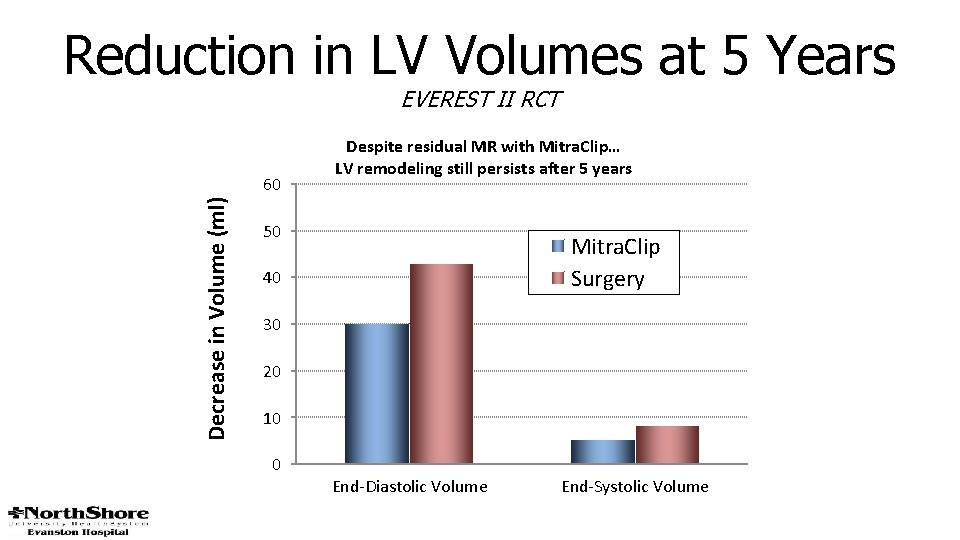
Reduction in LV Volumes at 5 Years EVEREST II RCT Decrease in Volume (ml) 60 Despite residual MR with Mitra. Clip… LV remodeling still persists after 5 years 50 Mitra. Clip Surgery 40 30 20 10 0 End-Diastolic Volume End-Systolic Volume

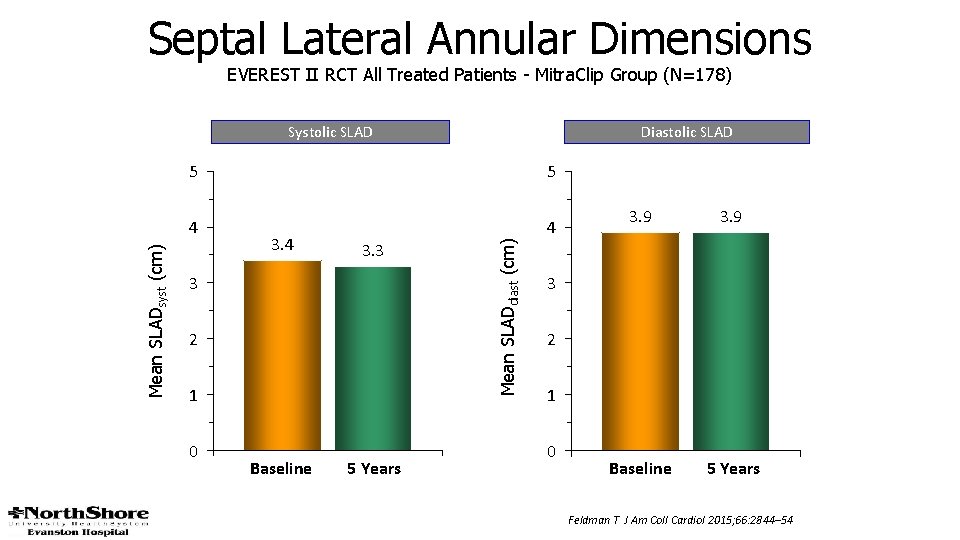
Septal Lateral Annular Dimensions EVEREST II RCT All Treated Patients - Mitra. Clip Group (N=178) Diastolic SLAD 5 5 4 4 3. 3 3 2 1 0 Baseline 5 Years Mean SLADdiast (cm) Mean SLADsyst (cm) Systolic SLAD 3. 9 Baseline 5 Years 3 2 1 0 Feldman T J Am Coll Cardiol 2015; 66: 2844– 54

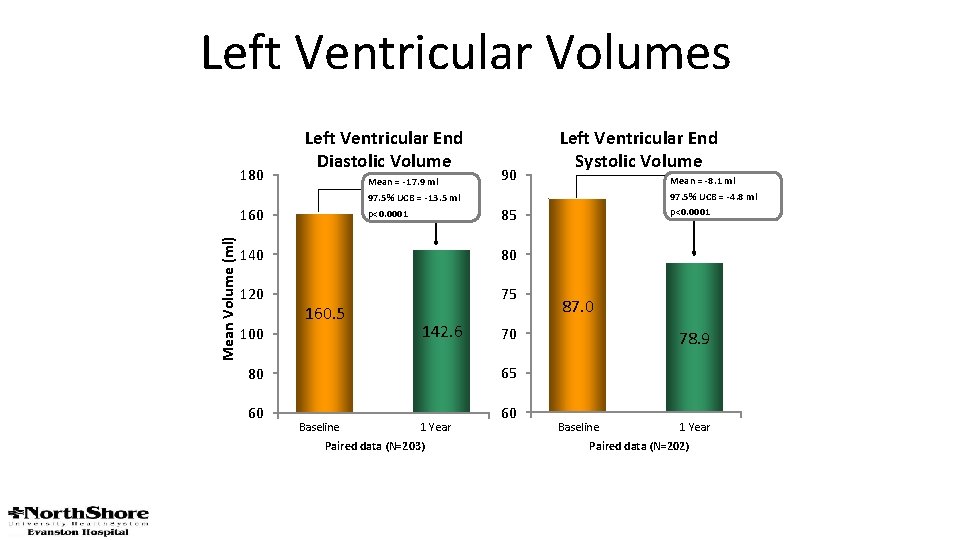
The percutaneous mitral valve device significantly reduced MR, improved clinical symptoms, and decreased LV dimensions at 12 months in this high-surgical-risk cohort. J Am Coll Cardiol 2014; 64: 172– 81

Left Ventricular Volumes 180 Left Ventricular End Diastolic Volume Mean = -17. 9 ml 97. 5% UCB = -13. 5 ml p<0. 0001 Mean Volume (ml) 160 90 80 120 75 100 142. 6 87. 0 70 78. 9 65 80 60 Mean = -8. 1 ml 97. 5% UCB = -4. 8 ml p<0. 0001 85 140 160. 5 Left Ventricular End Systolic Volume Baseline 1 Year Paired data (N=203) 60 Baseline 1 Year Paired data (N=202)

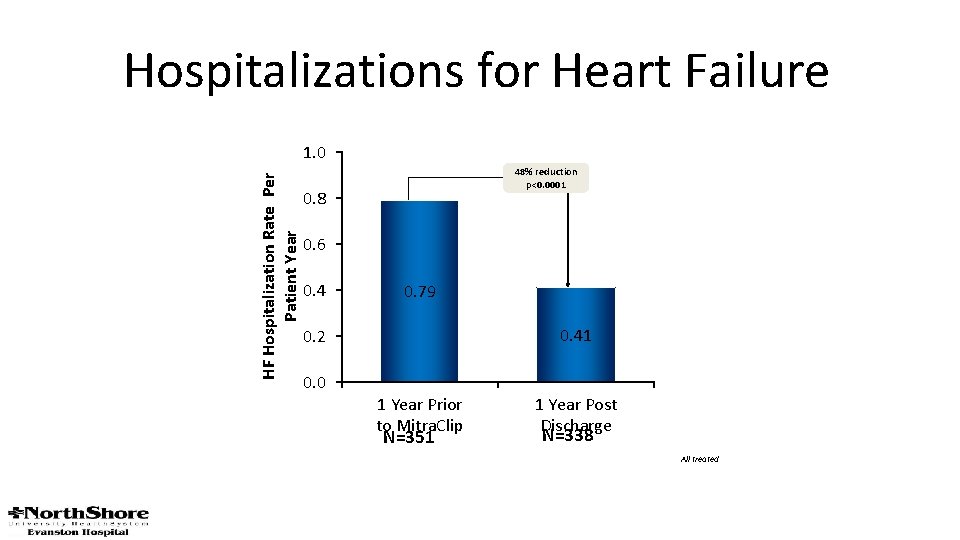
Hospitalizations for Heart Failure HF Hospitalization Rate Per Patient Year 1. 0 48% reduction p<0. 0001 0. 8 0. 6 0. 4 0. 79 0. 41 0. 2 0. 0 1 Year Prior to Mitra. Clip N=351 1 Year Post Discharge N=338 All treated

TMVR in prohibitive surgical risk patients is associated with safety and good clinical outcomes, including decreases in rehospitalization, functional improvements, and favorable ventricular remodeling, at 1 year. J Am Coll Cardiol 2014; 64: 182– 92

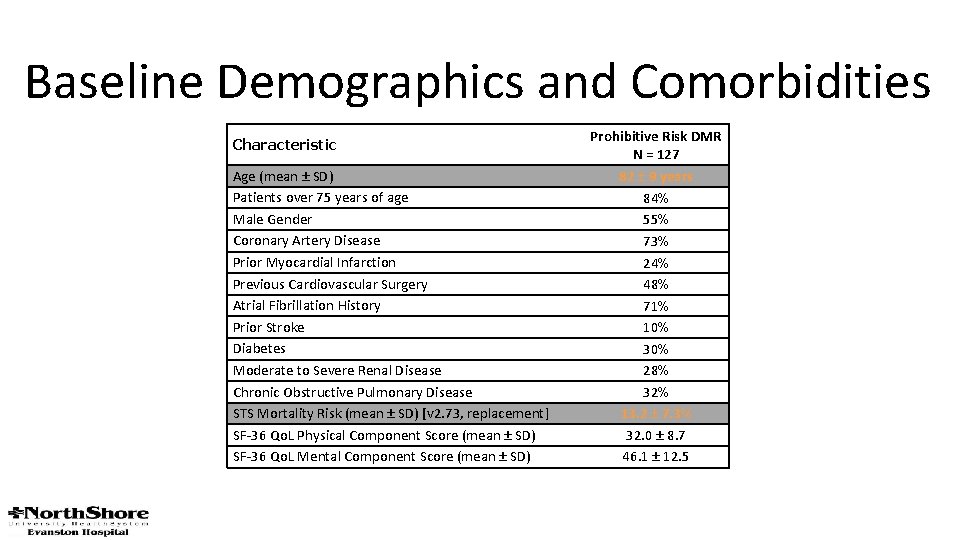
Baseline Demographics and Comorbidities Characteristic Age (mean ± SD) Patients over 75 years of age Male Gender Coronary Artery Disease Prior Myocardial Infarction Previous Cardiovascular Surgery Atrial Fibrillation History Prior Stroke Diabetes Moderate to Severe Renal Disease Chronic Obstructive Pulmonary Disease STS Mortality Risk (mean ± SD) [v 2. 73, replacement] SF-36 Qo. L Physical Component Score (mean ± SD) SF-36 Qo. L Mental Component Score (mean ± SD) Prohibitive Risk DMR N = 127 82 ± 9 years 84% 55% 73% 24% 48% 71% 10% 30% 28% 32% 13. 2 ± 7. 3% 32. 0 ± 8. 7 46. 1 ± 12. 5

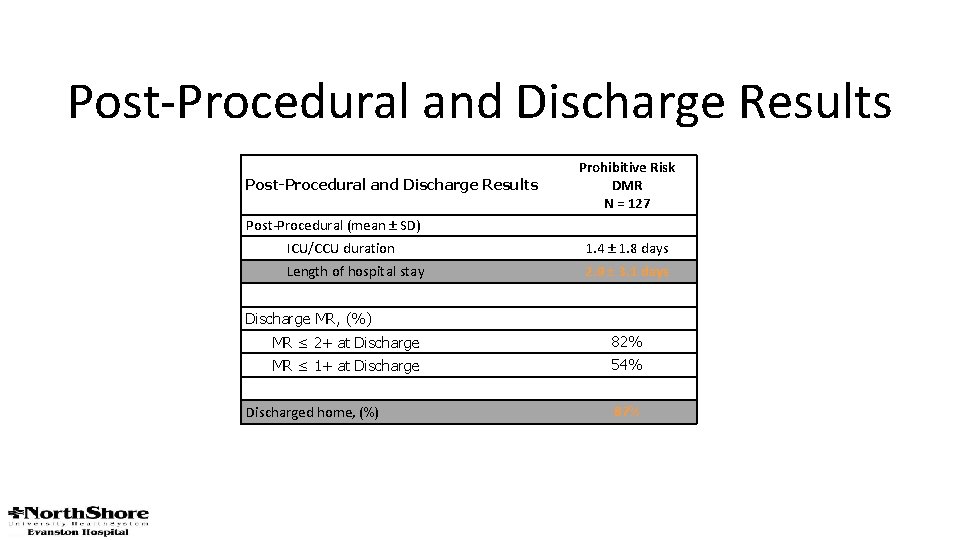
Post-Procedural and Discharge Results Prohibitive Risk DMR N = 127 Post-Procedural (mean ± SD) ICU/CCU duration 1. 4 ± 1. 8 days Length of hospital stay 2. 9 ± 3. 1 days Discharge MR, (%) MR ≤ 2+ at Discharge 82% MR ≤ 1+ at Discharge 54% Discharged home, (%) 87%

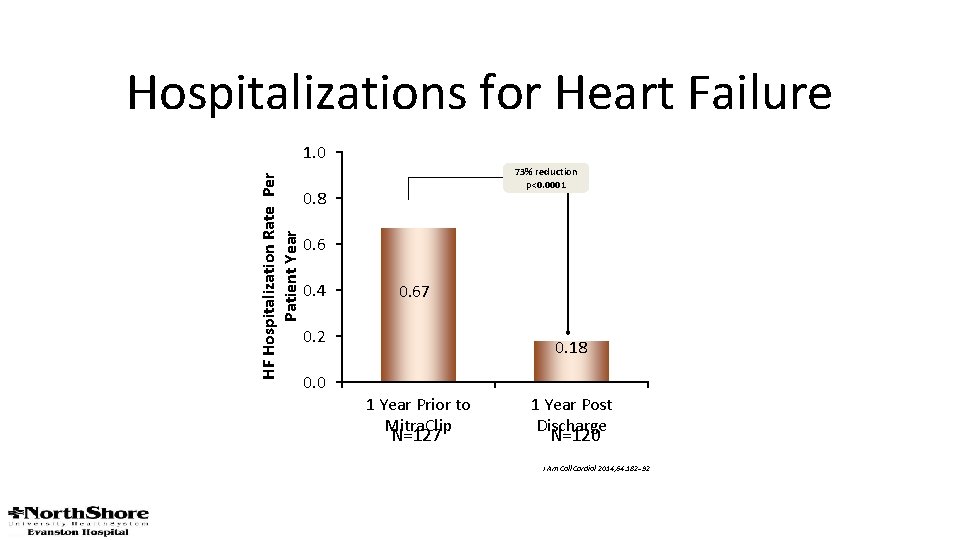
Hospitalizations for Heart Failure HF Hospitalization Rate Per Patient Year 1. 0 73% reduction p<0. 0001 0. 8 0. 6 0. 4 0. 67 0. 2 0. 0 0. 18 1 Year Prior to Mitra. Clip N=127 1 Year Post Discharge N=120 J Am Coll Cardiol 2014; 64: 182– 92

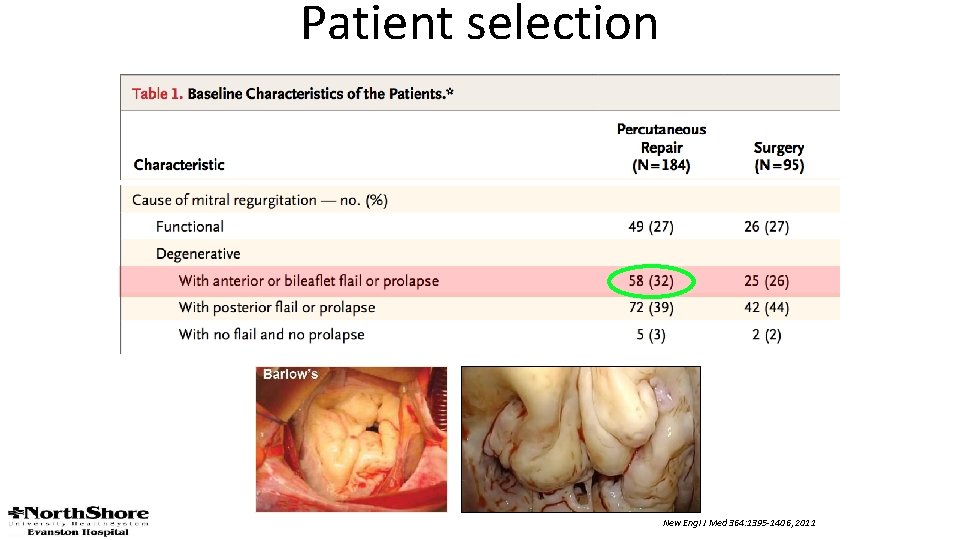
Patient selection New Engl J Med 364: 1395 -1406, 2011

The EVEREST II REALISM Continued Access Non-High Risk Study: Mid- and Long-Term Follow-up in Surgical Candidates Ted Feldman MD, Saibal Kar MD, D Scott Lim MD, Richard Smalling MD, Brian Whisenant MD, Chad Rammohan MD, Peter Fail MD, Michael Rinaldi MD, James Hermiller MD, Howard Herrmann MD, Robert Kipperman MD, James Slater MD, Elyse Foster MD, Neil J. Weissman MD, and Donald Glower MD On behalf of the EVEREST II Investigators ESC 2017 August 28, 2017 Study funded by Abbott Vascular. Investigators received research support from Abbott Vascular.

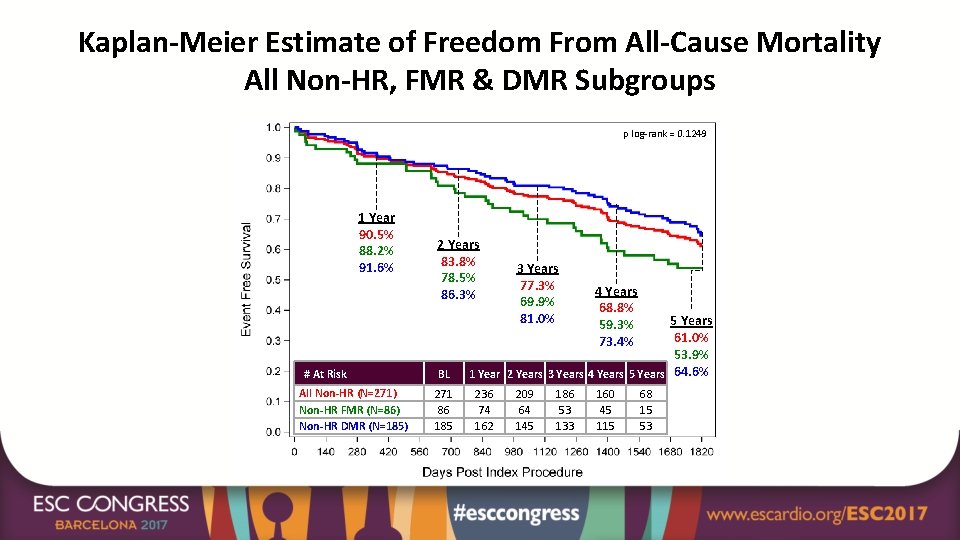
Kaplan-Meier Estimate of Freedom From All-Cause Mortality All Non-HR, FMR & DMR Subgroups p log-rank = 0. 1249 1 Year 90. 5% 88. 2% 91. 6% # At Risk All Non-HR (N=271) Non-HR FMR (N=86) Non-HR DMR (N=185) 2 Years 83. 8% 78. 5% 86. 3% BL 271 86 185 3 Years 77. 3% 69. 9% 81. 0% 4 Years 68. 8% 59. 3% 73. 4% 5 Years 61. 0% 53. 9% 1 Year 2 Years 3 Years 4 Years 5 Years 64. 6% 236 74 162 209 64 145 186 53 133 160 45 115 68 15 53

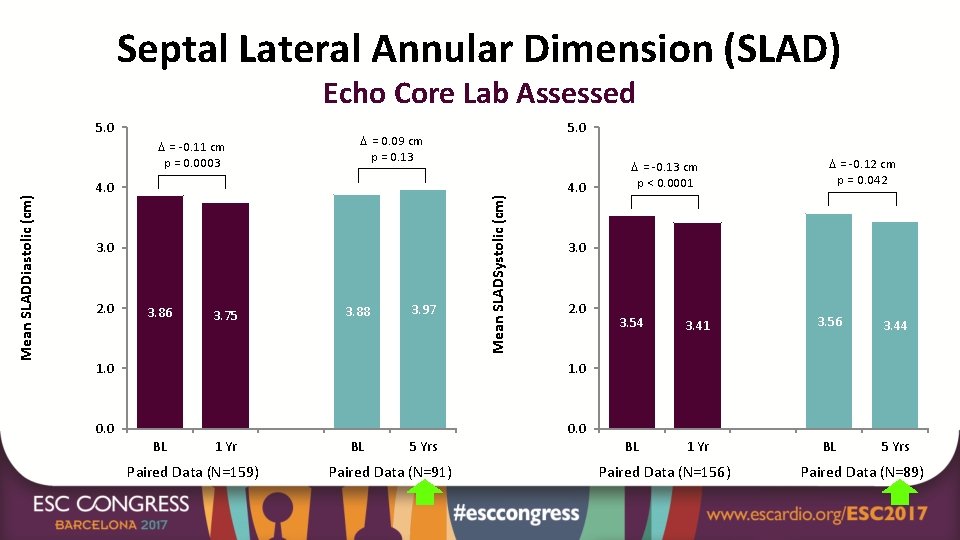
Septal Lateral Annular Dimension (SLAD) Echo Core Lab Assessed Mean SLADDiastolic (cm) = -0. 11 cm p = 0. 0003 = 0. 09 cm p = 0. 13 4. 0 3. 0 2. 0 3. 86 3. 75 5. 0 3. 88 3. 97 Mean SLADSystolic (cm) 5. 0 4. 0 2. 0 1. 0 0. 0 1 Yr Paired Data (N=159) BL 5 Yrs Paired Data (N=91) = -0. 12 cm p = 0. 042 3. 0 1. 0 BL = -0. 13 cm p < 0. 0001 3. 54 3. 41 3. 56 3. 44 BL 1 Yr BL 5 Yrs Paired Data (N=156) Paired Data (N=89)
 Pt. among mitra bakti utama
Pt. among mitra bakti utama Chiranjib mitra iiser kolkata
Chiranjib mitra iiser kolkata Pt dmh
Pt dmh Bal mitra gram
Bal mitra gram Ashim mitra
Ashim mitra Chitra mitra
Chitra mitra Yayasan envigo
Yayasan envigo Types of cholera
Types of cholera Omofori
Omofori Mitra nejad
Mitra nejad Gereja postkonsilier
Gereja postkonsilier Que es mitra en la biblia
Que es mitra en la biblia Subarna mitra
Subarna mitra Dr debasis mitra
Dr debasis mitra Mitra purandare
Mitra purandare Mitra rocca
Mitra rocca Mitra janes
Mitra janes Dr rito mitra
Dr rito mitra Uday mitra iisc
Uday mitra iisc Rito mitra
Rito mitra Dr subrata mitra
Dr subrata mitra Mitra amini
Mitra amini