Classification of Enzymes 1 Oxidoreductases 2 Transferases 3
![Brønsted Equation Halogenation of Carbonyl Compounds v = k. OH[OH-][acetone] + k. B[B][acetone] slow Brønsted Equation Halogenation of Carbonyl Compounds v = k. OH[OH-][acetone] + k. B[B][acetone] slow](https://slidetodoc.com/presentation_image_h/3681fe48602ebe0ab49bc2e0fc8d9c02/image-10.jpg)
- Slides: 11

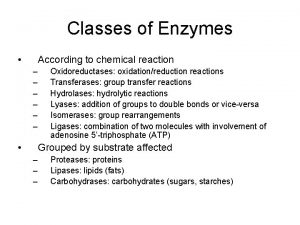
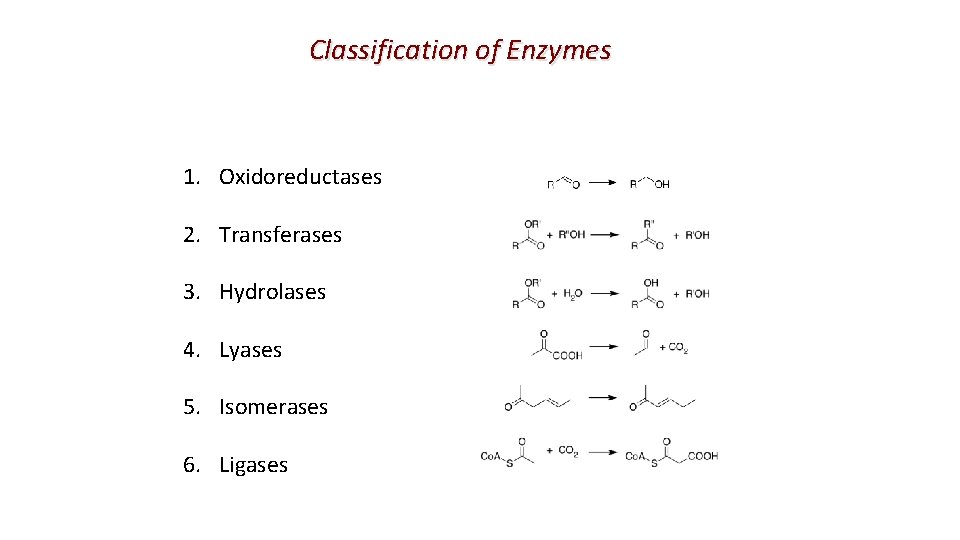
Classification of Enzymes 1. Oxidoreductases 2. Transferases 3. Hydrolases 4. Lyases 5. Isomerases 6. Ligases

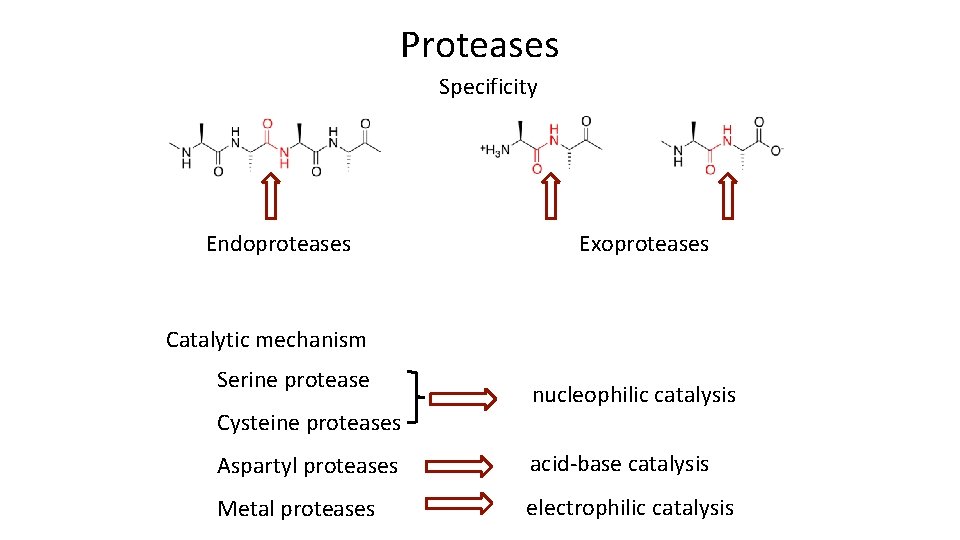
Proteases Specificity Endoproteases Exoproteases Catalytic mechanism Serine protease Cysteine proteases nucleophilic catalysis Aspartyl proteases acid-base catalysis Metal proteases electrophilic catalysis

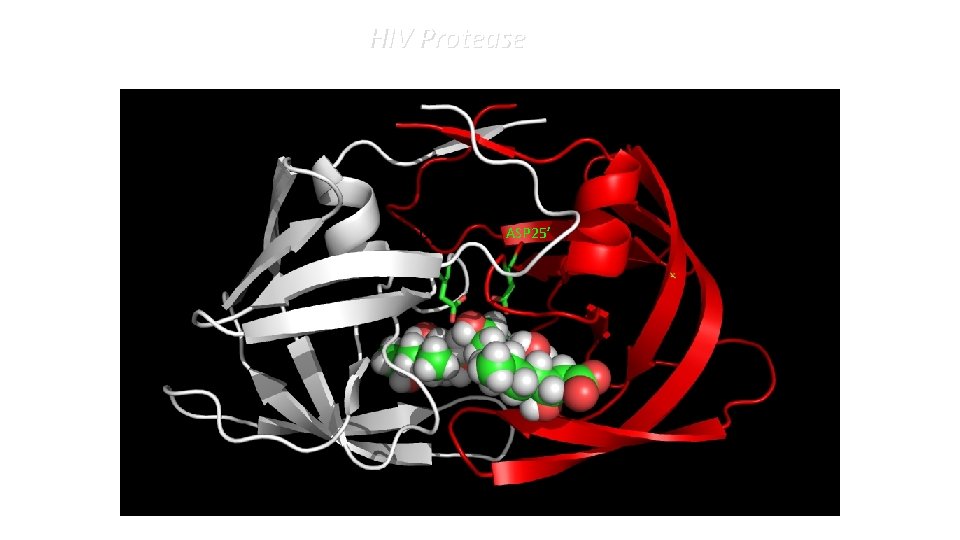
HIV Protease ASP 25’

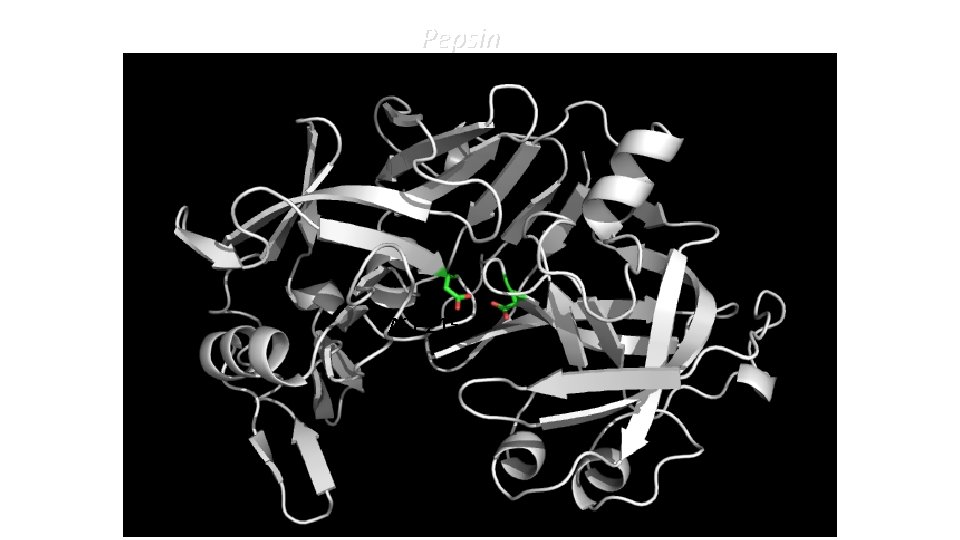
Pepsin ASP 215 ASP 32

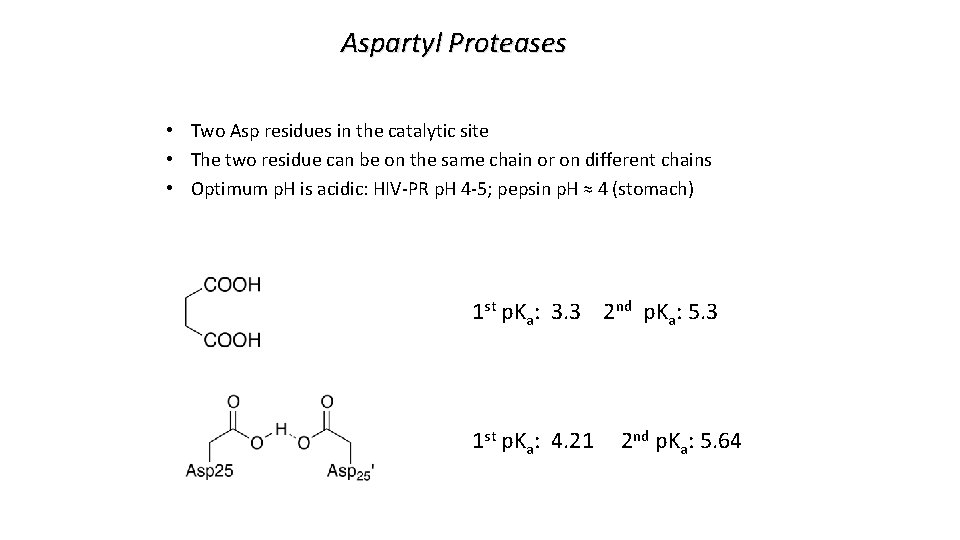
Aspartyl Proteases • Two Asp residues in the catalytic site • The two residue can be on the same chain or on different chains • Optimum p. H is acidic: HIV-PR p. H 4 -5; pepsin p. H ≈ 4 (stomach) 1 st p. Ka: 3. 3 2 nd p. Ka: 5. 3 1 st p. Ka: 4. 21 2 nd p. Ka: 5. 64

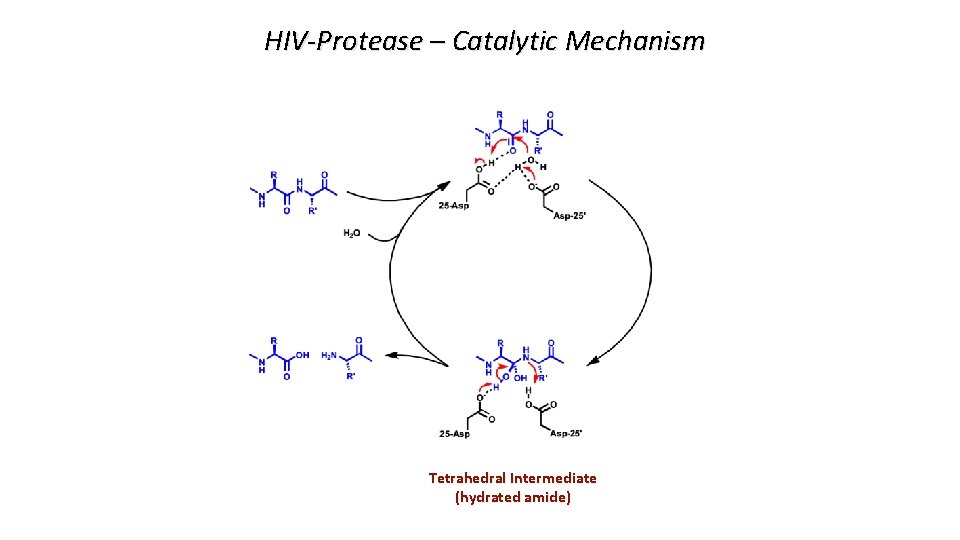
HIV-Protease – Catalytic Mechanism Tetrahedral Intermediate (hydrated amide)

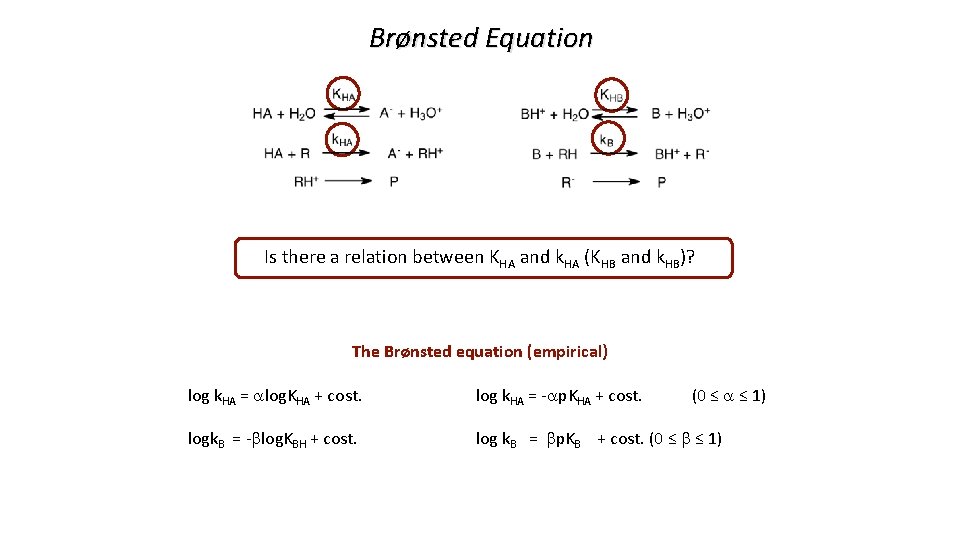
Brønsted Equation Is there a relation between KHA and k. HA (KHB and k. HB)? The Brønsted equation (empirical) log k. HA = alog. KHA + cost. log k. HA = -ap. KHA + cost. (0 ≤ a ≤ 1) logk. B = -blog. KBH + cost. log k. B = bp. KB + cost. (0 ≤ b ≤ 1)

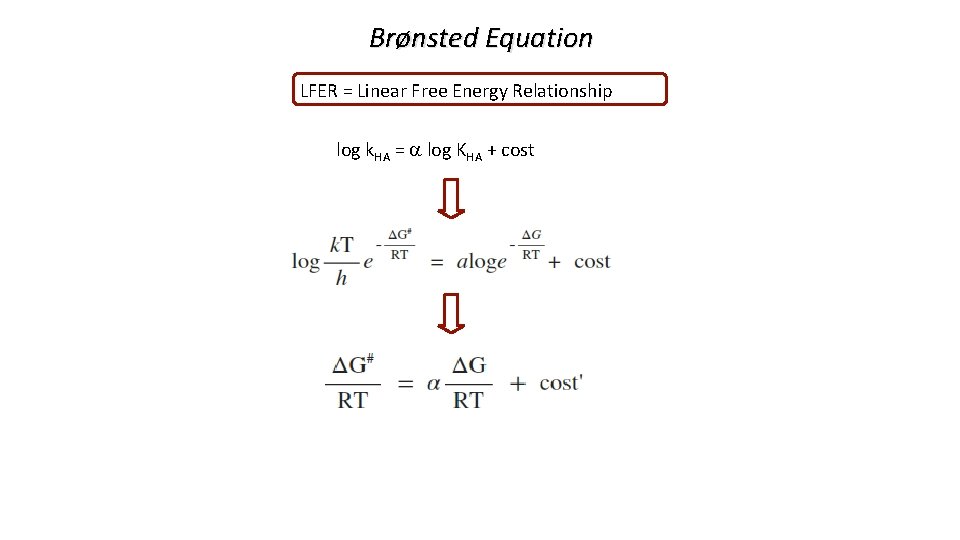
Brønsted Equation LFER = Linear Free Energy Relationship log k. HA = a log KHA + cost

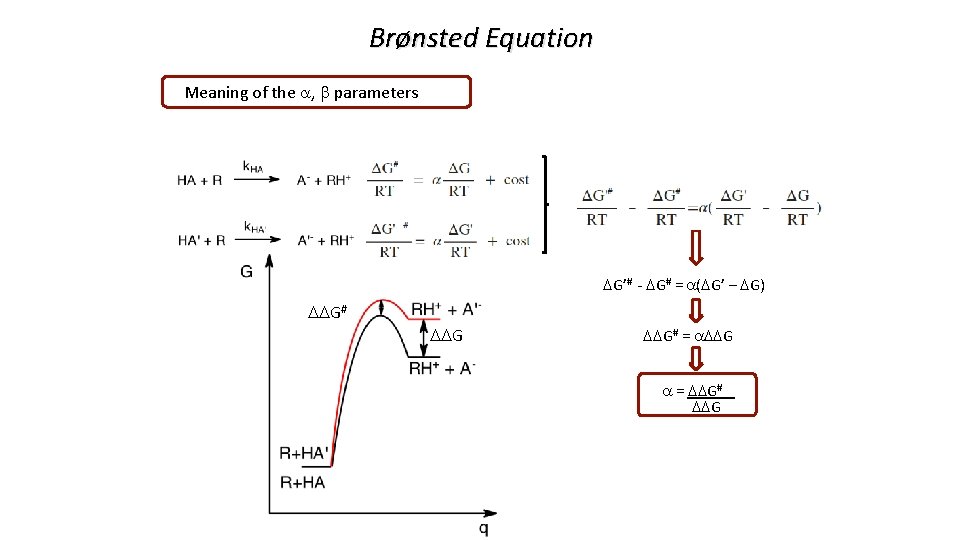
Brønsted Equation Meaning of the a, b parameters DG’# - DG# = a(DG’ – DG) DDG# = a. DDG a = DDG# DDG
![Brønsted Equation Halogenation of Carbonyl Compounds v k OHOHacetone k BBacetone slow Brønsted Equation Halogenation of Carbonyl Compounds v = k. OH[OH-][acetone] + k. B[B][acetone] slow](https://slidetodoc.com/presentation_image_h/3681fe48602ebe0ab49bc2e0fc8d9c02/image-10.jpg)
Brønsted Equation Halogenation of Carbonyl Compounds v = k. OH[OH-][acetone] + k. B[B][acetone] slow v = k 1[B][acetone] fast Hammond Postulate log k. B G b=0. 48 b=0. 88 p. KBH+ q

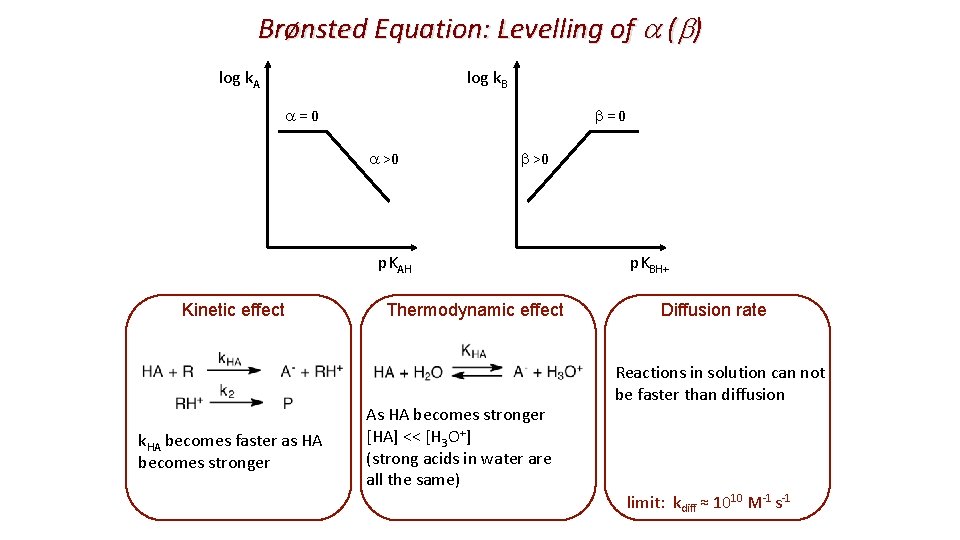
Brønsted Equation: Levelling of a (b) log k. B log k. A b=0 a >0 b >0 p. KAH Kinetic effect k. HA becomes faster as HA becomes stronger Thermodynamic effect As HA becomes stronger [HA] << [H 3 O+] (strong acids in water are all the same) p. KBH+ Diffusion rate Reactions in solution can not be faster than diffusion limit: kdiff ≈ 1010 M-1 s-1
 Examples of transferases
Examples of transferases Enzymes
Enzymes Classification of coenzyme
Classification of coenzyme Enzymes classification
Enzymes classification Classification of enzymes
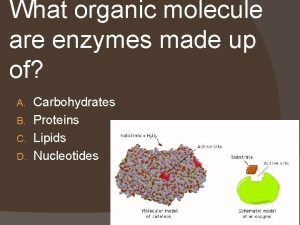
Classification of enzymes Enzymes are composed of what organic molecule
Enzymes are composed of what organic molecule Chief factory for digestive enzymes
Chief factory for digestive enzymes Immobilized enzymes
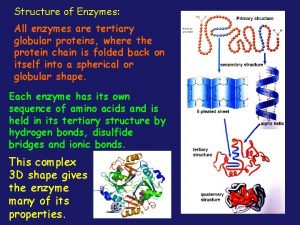
Immobilized enzymes All enzymes are globular proteins
All enzymes are globular proteins Function of restriction enzymes
Function of restriction enzymes Non functional plasma enzymes
Non functional plasma enzymes Prosthetic group
Prosthetic group