Chemistry Chemistry is the science concerned with the












- Slides: 17

Chemistry • Chemistry is the science concerned with the properties of matter and the changes in matter. • A chemist is one who studies the properties and changes in matter.

Matter • Matter is anything that has mass and takes up space.

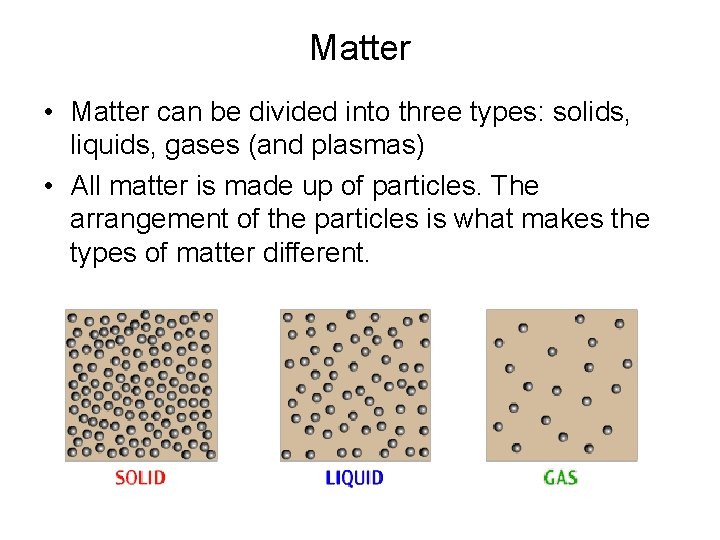
Matter • Matter can be divided into three types: solids, liquids, gases (and plasmas) • All matter is made up of particles. The arrangement of the particles is what makes the types of matter different.


Solids • The particles in a solid are close together. They are unable to move past each other. • The particles are strongly attracted to each other. • A solid does not take the shape of its container. • An example is an ice cube in a glass.

Liquids • The particles in a liquid are close, but not as close as in a solid. • The particles can slide past each other because they are less attracted to each other. • A liquid takes the shape of its container. And example is pop in a can or juice in a glass.

• Some particles flow more easily than others. • The resistance to flow is called viscosity. • Honey and molasses have high viscosity • Water has a low viscosity.

Gases • The particles in a gas are far apart resulting in a lot of movement. They are not strongly attracted to each other and are very fast-moving. • Due to the amount of space between particles, it is possible to compress a gas.


Plasmas • The fourth state of matter is plasma. Plasma is very rare on earth, but is one of the most common states in the universe. • Plasma has high levels of energy and cannot be contained by any object on our earth composed of ordinary matter.

Phase Changes • There are six phase changes. – Solid to liquid is called melting. – Liquid to solid is called freezing. – Liquid to gas is called evaporation. – Gas to liquid is called condensation. – Solid to gas, without going through the liquid phase, is called sublimation. – Gas to solid, without going through the liquid phase is called deposition.


Properties of Matter • There are two characteristics or properties that help determine forms of matter – physical properties and chemical properties.

Physical Properties of Matter • Physical properties describe what the material is like. These are visible features that can be observed or measured. – – – – State – solid, liquid, gas, plasma Colour Density, viscosity Hardness, brittleness Taste, odour, texture, lustre, clarity Melting and boiling points Ductility (bendable) and malleability (able to be hammered)

• Physical properties can often be further classified as either qualitative or quantitative. – A qualitative physical property is a characteristic that can be described but not measured. – A quantitative physical property is a characteristic of a substance that can be measured numerically.


Chemical Properties of Matter • Chemical properties explain how a material behaves and reacts in relation to other materials. – Types of bonds – Reactivity – Isotopes formed
 Economics is the social science concerned with
Economics is the social science concerned with What is science concerned with?
What is science concerned with? Science is concerned with
Science is concerned with My favorite subject science
My favorite subject science Network layer design issues in computer networks
Network layer design issues in computer networks Branch of biology concerned with the study of fungi
Branch of biology concerned with the study of fungi Concerned with quantity
Concerned with quantity Biology is concerned with
Biology is concerned with Formal vs functional region
Formal vs functional region Cuss communication tool
Cuss communication tool Proper adjective for steinbeck
Proper adjective for steinbeck The procedural view of democracy is most concerned with
The procedural view of democracy is most concerned with Ethics is the branch of philosophy concerned with
Ethics is the branch of philosophy concerned with Cattell's 16 personality factors
Cattell's 16 personality factors Spencer rugaber
Spencer rugaber Union of concerned scientists
Union of concerned scientists Bookkeeping is mainly concerned with
Bookkeeping is mainly concerned with In structural decomposition are concerned
In structural decomposition are concerned