Chemistry Chapter 1 St Augustine Preparatory School 20152016












- Slides: 19

Chemistry Chapter 1 St. Augustine Preparatory School 2015/2016

What is chemistry? • Chemistry is the study of the composition, structure, and properties of matter, the processes that matter undergoes, and the energy changes that accompany the processes

There are numerous fields of chemistry • Organic chem: study of carbon containing compounds • Inorganic chem: study of non-carbon containing (non-organic) compounds • Physical chem: the study of properties and changes of matter and their relation to energy • Analytical chem: identification of the components and composition of materials

There are numerous fields of chemistry • Biochemistry: study of processes and substances occurring in living things • Theoretical chem: Using mathematics and computers to understand the principals behind observed chemical behaviours

Mass vs. Matter • Mass is a measure of the amount of matter • So then matter could be defined as:

Mass vs. Matter • Mass is a measure of the amount of matter • So then matter could be defined as: Anything that has mass and takes up space

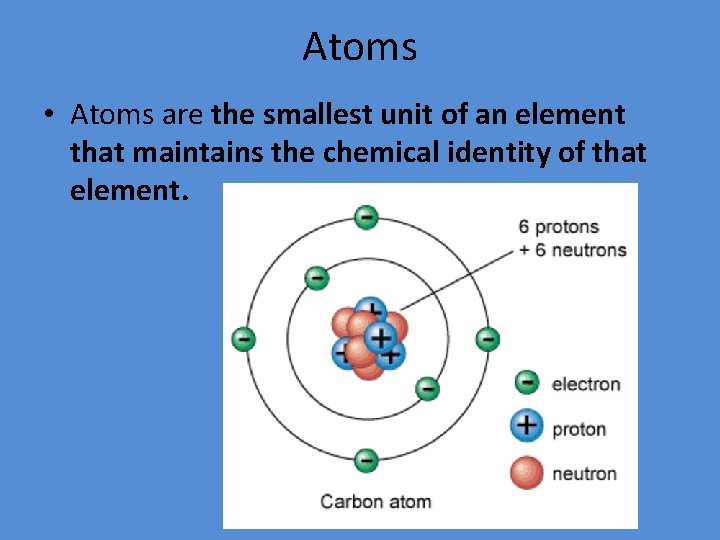
Atoms • Atoms are the smallest unit of an element that maintains the chemical identity of that element.

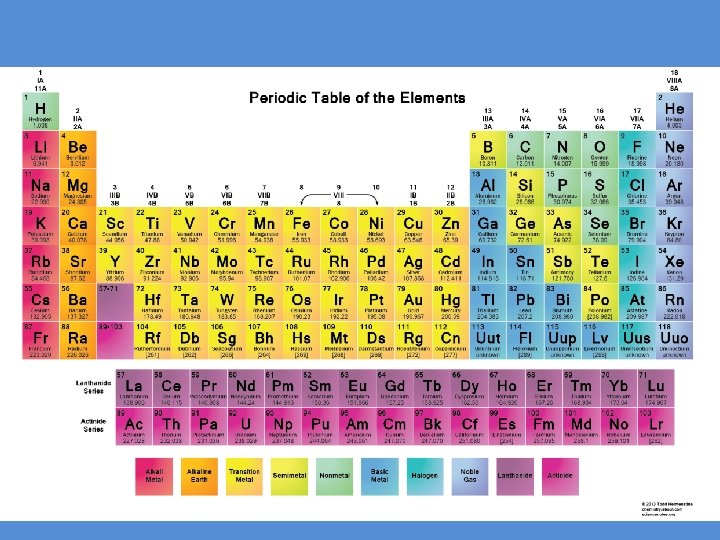
Elements • Elements are pure substances that cannot be broken down into simpler, stable substances and is made of only one type of atom • Many elements are diatomic, meaning they exist in pairs of two (HOFBr. INCl or the “ 7 that make a 7”) H: Hydrogen. O: Oxygen F: Fluorine Br: Bromine I: Iodine N: Nitrogen Cl: Chlorine – This will be very important later in this year!!

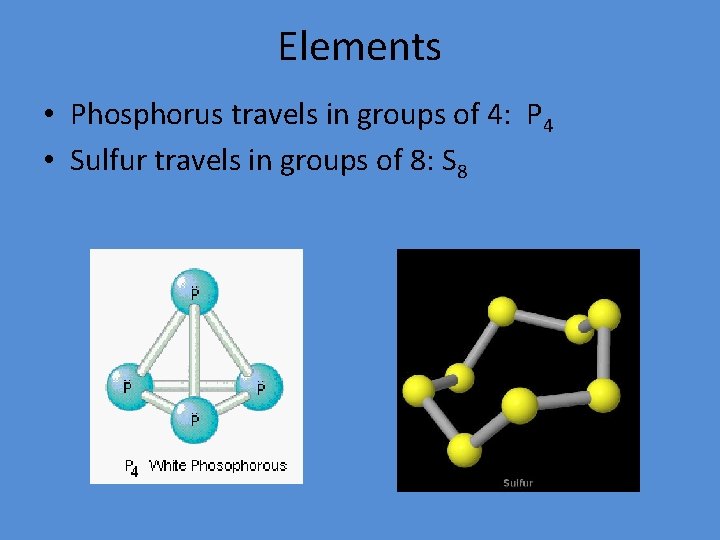
Elements • Phosphorus travels in groups of 4: P 4 • Sulfur travels in groups of 8: S 8


Substances have characteristic properties • Extensive Properties: depend on the amount of matter that is present. (Volume, mass, and the amount of energy) • Intensive properties: do not depend on the amount of matter (Boiling point, melting point, ability to conduct electricity)

Extensive or Intensive? 1) 2) 3) 4) There’s 32 grams of phosphorus in a beaker. Copper conducts electricity. Water boils at 100 degrees Celsius. A pot of water needed to be heated for 8 minutes before it boiled. 5) Elemental sulfur is bright yellow. 6) A 365 m. L can of coca cola contains 140 calories. 7) Melting a block of ice required 1. 6 k. J of energy.

Extensive or Intensive? 1) 2) 3) 4) There’s 32 grams of phosphorus in a beaker. Extensive Copper conducts electricity. Intensive Water boils at 100 degrees Celsius. Intensive A pot of water needed to be heated for 8 minutes before it boiled. Extensive 5) Elemental sulfur is bright yellow. Intensive 6) A 365 m. L can of coca cola contains 140 calories. Extensive 7) Melting a block of ice required 1. 6 k. J of energy. Extensive

There also physical and chemical properties • Physical properties: a characteristic that can be observed or measured without changing the identity of the substance – Boiling point – Melting point – Density – Color – Lustre

Physical Change • Any change that does not alter the identity of the substance

Changes to the states of matter are Physical Changes • In groups of 3, you will read about solids, liquids, and gases on page 8 and 9 of your textbook • Each individual in your group is responsible for reading one section and then explaining to their group what was mentioned in his/her section • Write down at least 3 important points for each of the three states of matter (gas, liquid, and solid) **Pay close attention to whether your state has a definite volume or takes the shape of a container**

Chemical Changes • When chemical changes occur, the actual composition of the chemical is changed and new substances are formed • Some signs of a chemical change (there are lots more): – Color change – Bubbling (from gas) in a reaction – Formation of a solid (precipitate) – Light is emitted

Are the following physical or chemical changes? 1) A hot glass shatters when put into cold water 2) Wood is burned in a fireplace 3) A nail in a piece of wood rusting 4) Tearing a piece of paper in half 5) Food molding 6) Perfume evaporating off of your skin 7) Butter melting 8) Moth balls vaporizing in a closet

Matter can be a pure substance or a mixture • Read pages 11, 12, and 13 on mixtures and pure substances. At the end, you should be able to sort different substances into: – Mixtures or Pure substances – Compounds, Elements, Heterogeneous mixtures, and Homogeneous mixtures