Chemistry Chapter 2 St Augustine Preparatory School August










- Slides: 15

Chemistry Chapter 2 St. Augustine Preparatory School August 7 Lecture

Observations • Observations can be qualitative or quantitative • Qualitative relates to a quality of something such as color, texture, shape – Something you see, feel, hear, etc. • Quantitative relates to quantity – something that is measured. Ie. 10 meters, 29. 5°C, 4 students, etc.

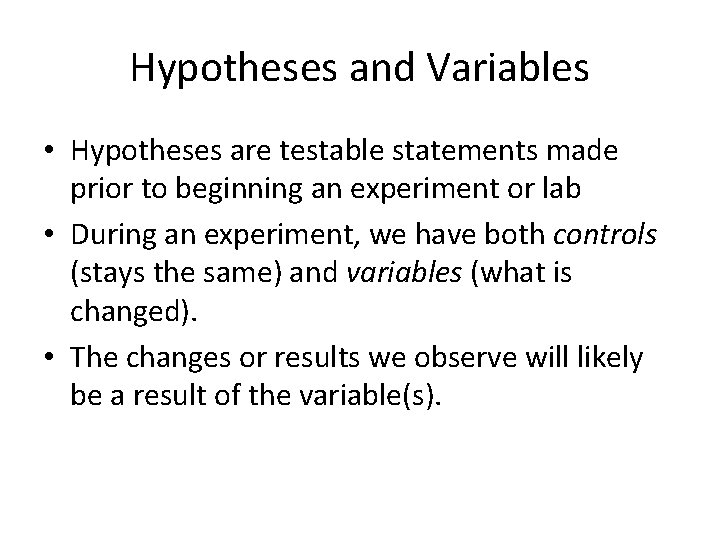
Hypotheses and Variables • Hypotheses are testable statements made prior to beginning an experiment or lab • During an experiment, we have both controls (stays the same) and variables (what is changed). • The changes or results we observe will likely be a result of the variable(s).

Scientific Method 1) Observations are made or current evidence is evaluated 2) A hypothesis must be made – what is expected to be found? 3) Testing – experiment, measure, and collect data 4) Theorizing – What did your data tell you? 5) Publish results – this allows others to benefit from your research

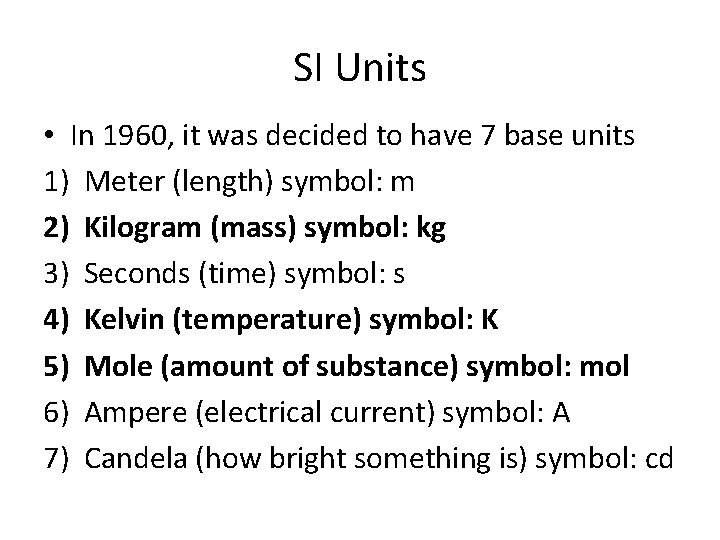
SI Units • In 1960, it was decided to have 7 base units 1) Meter (length) symbol: m 2) Kilogram (mass) symbol: kg 3) Seconds (time) symbol: s 4) Kelvin (temperature) symbol: K 5) Mole (amount of substance) symbol: mol 6) Ampere (electrical current) symbol: A 7) Candela (how bright something is) symbol: cd

Other units • There are more than these 7 units in science! – Units that come from a combination of the 7 base units, such as velocity (m/s), area (m 2), or volume (m 3) are called derived units • There also non-SI units that are widely accepted and used in science, such as Liters • 1 liter = 1000 cm 3

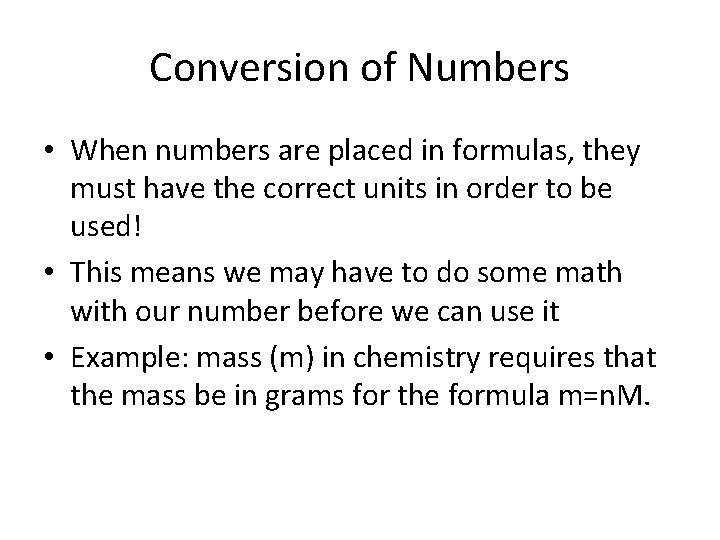
Conversion of Numbers • When numbers are placed in formulas, they must have the correct units in order to be used! • This means we may have to do some math with our number before we can use it • Example: mass (m) in chemistry requires that the mass be in grams for the formula m=n. M.

Conversions • There are 1000 milliliter in 1. 0 liter • There is 0. 001 liter in 1. 0 milliliter • There is 1000 mg in 1. 0 gram • There is 0. 001 grams in 1. 0 milligram • There is 1000 grams or liters in 1 kilogram or kiloliter • There is 0. 001 k. L or kg in 1 L or 1 g

Conversion of Numbers • Example 1: Convert 0. 321 kg to grams • Example 2: Convert 32. 67 m. L to liters

Try these on your own 1) 132. 7 milligrams to grams 1) 0. 00325 kilograms to milligrams 1) 235 grams in kilograms 1) 1 345. 9 milliliters to liters 1) 0. 68 kiloliters in liters 2) 0. 003 kiloliters in milliliters

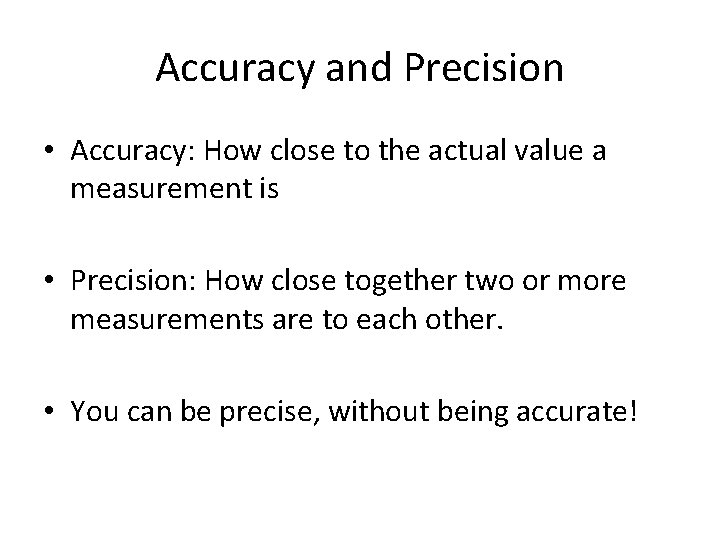
Accuracy and Precision • Accuracy: How close to the actual value a measurement is • Precision: How close together two or more measurements are to each other. • You can be precise, without being accurate!

Consider the game of darts, if a person is aiming at the center of the board.

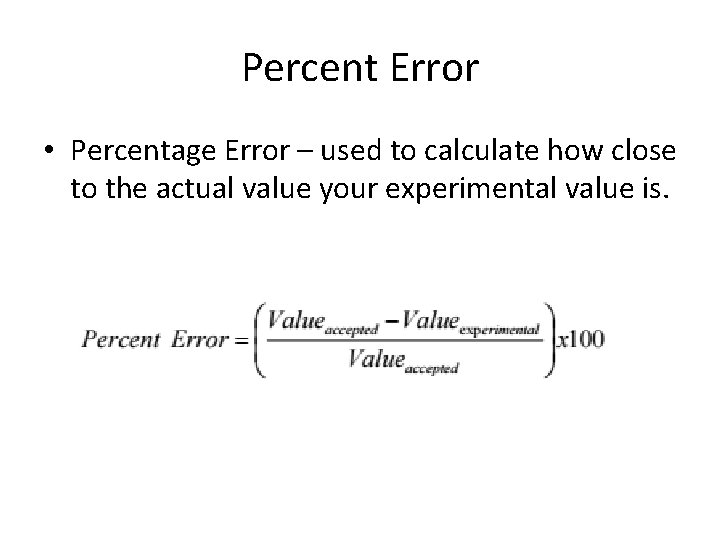
Percent Error • Percentage Error – used to calculate how close to the actual value your experimental value is.

Percent Error Questions • You are given a water sample that contains 2. 304 mg of Mercury. You run tests on it, and conclude that it was 2. 516 mg of mercury. What is your percent error?

Percent Error Questions • You receive another water sample and are told that it contains 0. 00325 g of phosphorous. After testing the sample, you find that it has 3. 79 mg of phosphorous. What is the percent error?