Physics Chapter 1 St Augustine Preparatory School The














- Slides: 17

Physics Chapter 1 St. Augustine Preparatory School

The Scientific Method • Physics follows the scientific method 1) Make observations and collect data - This will allow you to formulate a question 2) Create and test a hypothesis through controlled experimentation 3) Interpret results and revise hypothesis if necessary 4) State conclusions in a form that can be evaluated by others

Using Models • Physics can often be very complex, so simplified models can be used • System – the interacting components considered to be necessary for the purpose of the study

• Example: Improving the fuel efficiency of a vehicle. Things that could potentially matter:

• To run a legitimate and effective study, we cannot change all of these variables at once. • We must also run a “control” group that has only changed one variable. • The best physics models can make predictions in new situations

If we drop a roll of tape and a piece of paper at the same time, which one is going to hit the ground first? What about in a vacuum? (youtube video) What can we conclude from both of these trials?

SI Units In 1960, an international committee decided on a system of standards and units, known as SI units (Systeme International d’Unites). We have 7 base units in SI: 1) meter 2) kilogram 3) second 4) ampere 5) candela 6) kelvin and 7) mole

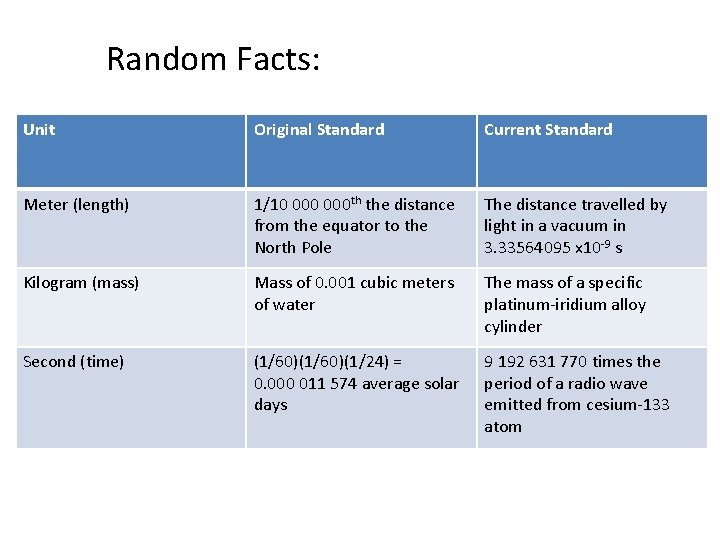
Random Facts: Unit Original Standard Current Standard Meter (length) 1/10 000 th the distance from the equator to the North Pole The distance travelled by light in a vacuum in 3. 33564095 x 10 -9 s Kilogram (mass) Mass of 0. 001 cubic meters of water The mass of a specific platinum-iridium alloy cylinder Second (time) (1/60)(1/24) = 0. 000 011 574 average solar days 9 192 631 770 times the period of a radio wave emitted from cesium-133 atom

These 7 cannot explain everything though… These units have been combined to create what we call “derived units” Example: Velocity is measured in m/s (meters per second) However, force is measured in N (Newton’s)

Prefixes for units You are required to know the following prefixes for units: a) Pico (p): 10 -12 g) kilo(k): 103 b) Nano(n): 10 -9 h) mega(M): 106 c) Micro(μ): 10 -6 i) giga(G): 109 d) Milli(m): 10 -3 j) tetra(T): 1012 e) Centi(c): 10 -2 f) deci(d): 10 -1

Conversions • Example: Convert 162000 g to Mg • Example: Convert 1312 km to meters

Practice conversions 1) 10 kilograms in megagrams 2) 0. 00342 kilometers in centimeters 3) 132. 5 cm in decimeters 4) 132 millimeters to micrometers 5) 262, 523 micrometers in meters 6) 2329 picograms to milligrams

Significant Figures • Significant figures (also called significant digits) are important as they indicate the precision of a measurement • Page 18 in your textbook has the rules for determining the amount of significant figures in a number

Practice a) b) c) d) e) f) g) 1. 0303 m 2. 50 kg 0. 00032 s 3. 0 x 102 mm 45 s 40 s 2. 2830 km h) 1000 kg i) 6. 723 m j) 4. 200 J

Rules for addition and subtraction • When adding and subtracting numbers, the final answer will have the same number of decimal places as the number used in the addition/subtraction that had the least number of decimal places. • Examples: 121. 03 + 5. 8 = 125 – 5. 5 =

Rules for multiplication and division • When multiplying and dividing, the final answer must have the same number of significant figures as the number used that had the least number of significant digits. Examples: a) 24. 0 x 1. 5 = b) 36. 00/2. 00 =

Practice 1) 2) 3) 4) 5) 6) 7) 8) 97. 3 + 5. 85 = 122. 3 – 0. 52 = (124. 2)(2. 1) = (2 x 102)(1. 232) = 24. 56 / 2. 3213 = 2. 02 x 106 + 1. 3 x 105 = 1. 000 + 2. 01 + 3. 523 + 2. 6 = (1. 20)(2. 212)(0. 0035) =