CHEMICAL CHANGE 17 1 Chapter Seventeen Chemical Change








- Slides: 13

CHEMICAL CHANGE 17. 1

Chapter Seventeen: Chemical Change Ø 17. 1 Chemical Reactions Ø 17. 2 Balancing Equations Ø 17. 3 Classifying Reactions

Chapter 17. 1 Learning Goals ØDescribe how energy is involved in chemical changes. ØIdentify evidence that a chemical change has occurred. ØExplain what happens during chemical reactions.

Investigation 17 A Chemical Equations ØKey Question: How are atoms conserved in a chemical reaction?

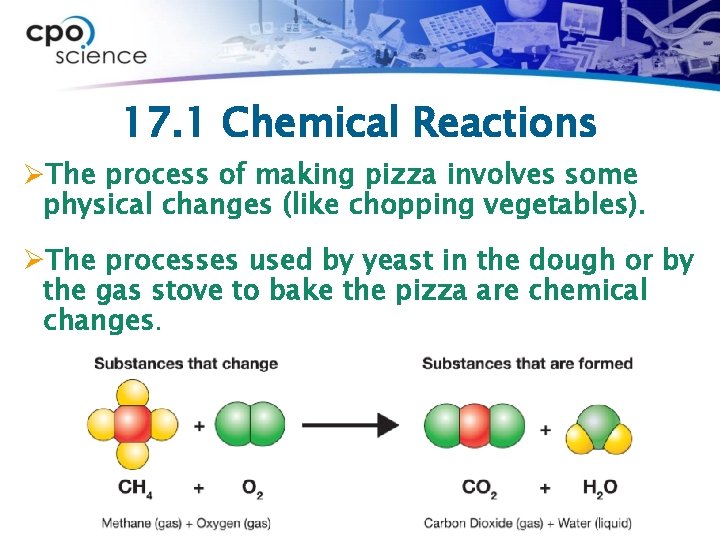
17. 1 Chemical Reactions ØA chemical reaction is the process of breaking of chemical bonds in one or more substances, and the reforming of new bonds to create new substances. ØWhen you make pizza, which changes are physical and which are chemical changes?

17. 1 Chemical Reactions ØThe process of making pizza involves some physical changes (like chopping vegetables). ØThe processes used by yeast in the dough or by the gas stove to bake the pizza are chemical changes.

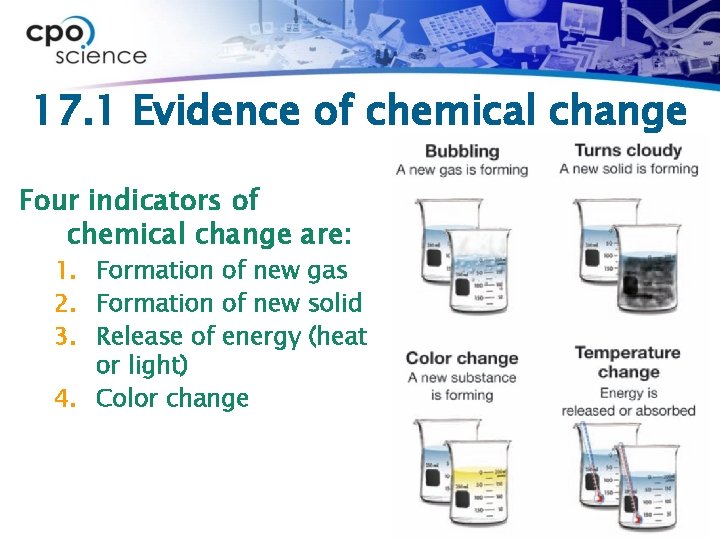
17. 1 Evidence of chemical change Four indicators of chemical change are: 1. Formation of new gas 2. Formation of new solid 3. Release of energy (heat or light) 4. Color change


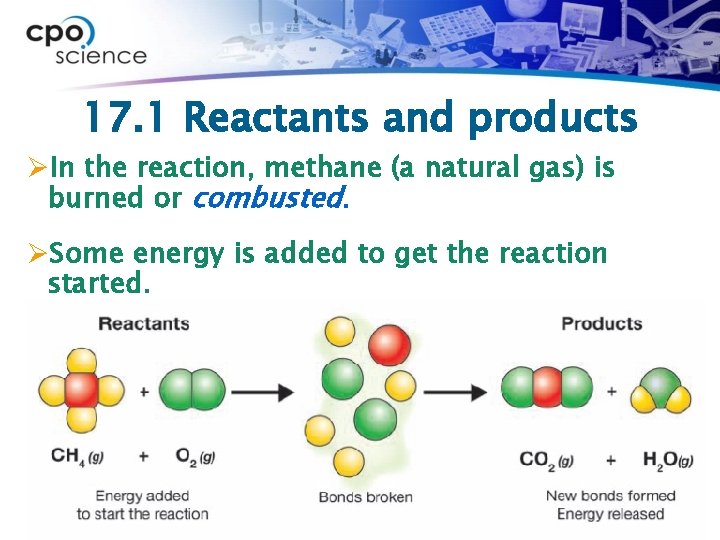
17. 1 Products and reactants Ø In chemical reactions, you start with reactants that are combined to make products. Ø The reactants are the starting substances. Ø The products are the new substances which result from the chemical reaction.


17. 1 Reactants and products ØIn the reaction, methane (a natural gas) is burned or combusted. ØSome energy is added to get the reaction started.


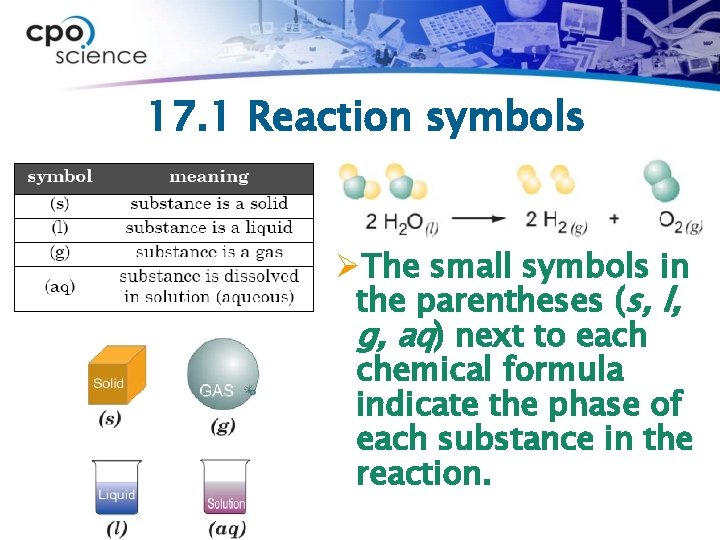
17. 1 Reaction symbols ØThe small symbols in the parentheses (s, l, g, aq) next to each chemical formula indicate the phase of each substance in the reaction.
 Dance moms seventeen magazine
Dance moms seventeen magazine Seventeen master key
Seventeen master key Seventeen current manager
Seventeen current manager Seventeen table
Seventeen table Painting a wall chemical or physical change
Painting a wall chemical or physical change Meaning of physical change
Meaning of physical change Difference between a chemical and physical change
Difference between a chemical and physical change Physical change
Physical change Spare change physical versus chemical change
Spare change physical versus chemical change Whats the difference between a chemical and physical change
Whats the difference between a chemical and physical change How does a physical change differ from a chemical change
How does a physical change differ from a chemical change How does a physical change differ from a chemical change?
How does a physical change differ from a chemical change? Is chopping wood physical or chemical change
Is chopping wood physical or chemical change Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds
























