CHEMICAL CHANGE Chapter Seventeen Chemical Change 17 1
































- Slides: 60

CHEMICAL CHANGE

Chapter Seventeen: Chemical Change Ø 17. 1 Chemical Reactions Ø 17. 2 Balancing Equations Ø 17. 3 Classifying Reactions

Chapter 17. 1 Learning Goals ØDescribe how energy is involved in chemical changes. ØIdentify evidence that a chemical change has occurred. ØExplain what happens during chemical reactions.

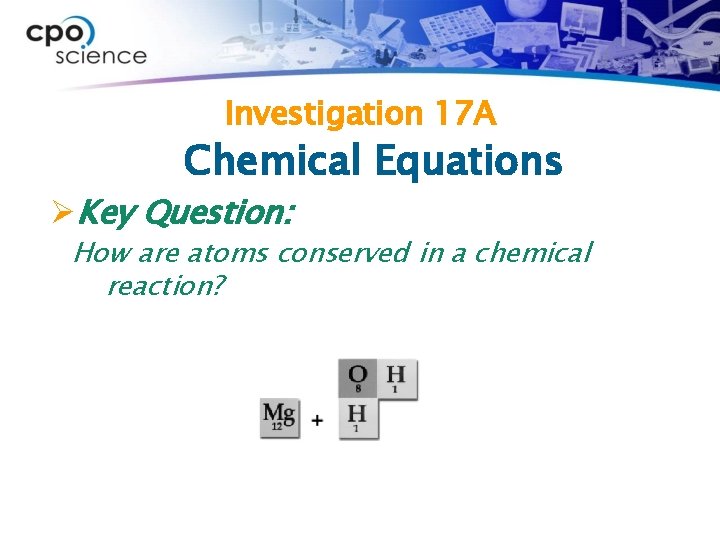
Investigation 17 A Chemical Equations ØKey Question: How are atoms conserved in a chemical reaction?

17. 1 Chemical Reactions ØA chemical reaction is the process of breaking of chemical bonds in one or more substances, and the reforming of new bonds to create new substances. ØWhen you make pizza, which changes are physical and which are chemical changes?

17. 1 Chemical Reactions ØThe process of making pizza involves some physical changes (like chopping vegetables). ØThe processes used by yeast in the dough or by the gas stove to bake the pizza are chemical changes.

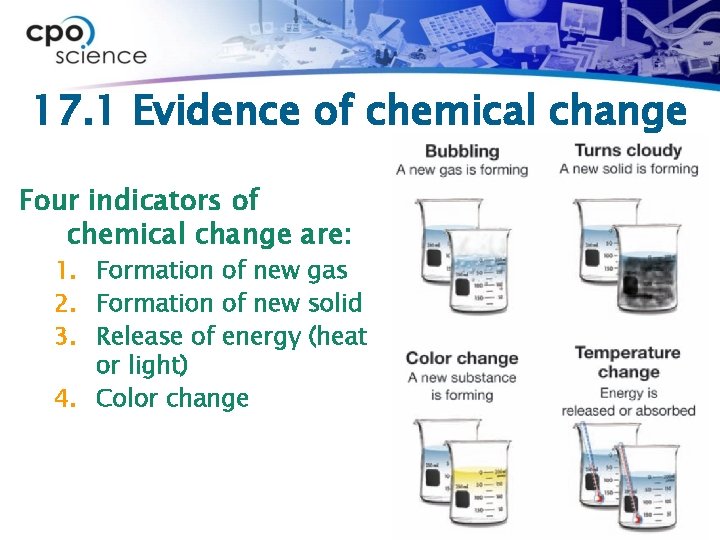
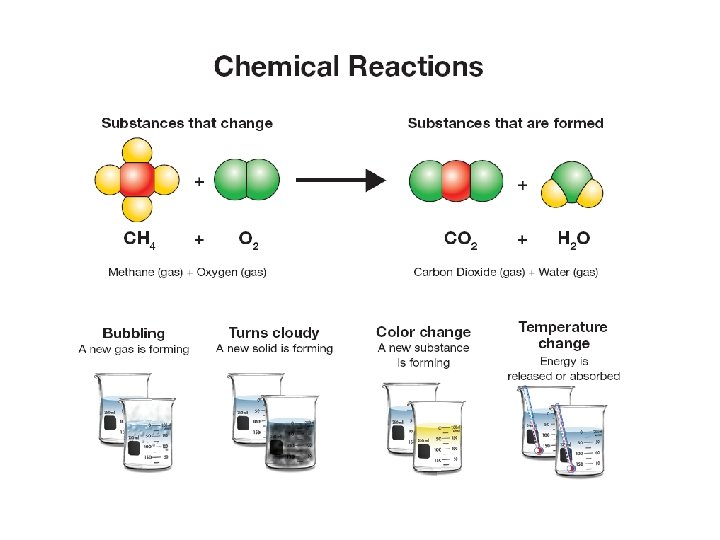
17. 1 Evidence of chemical change Four indicators of chemical change are: 1. Formation of new gas 2. Formation of new solid 3. Release of energy (heat or light) 4. Color change


17. 1 Products and reactants Ø In chemical reactions, you start with reactants that are combined to make products. Ø The reactants are the starting substances. Ø The products are the new substances which result from the chemical reaction.


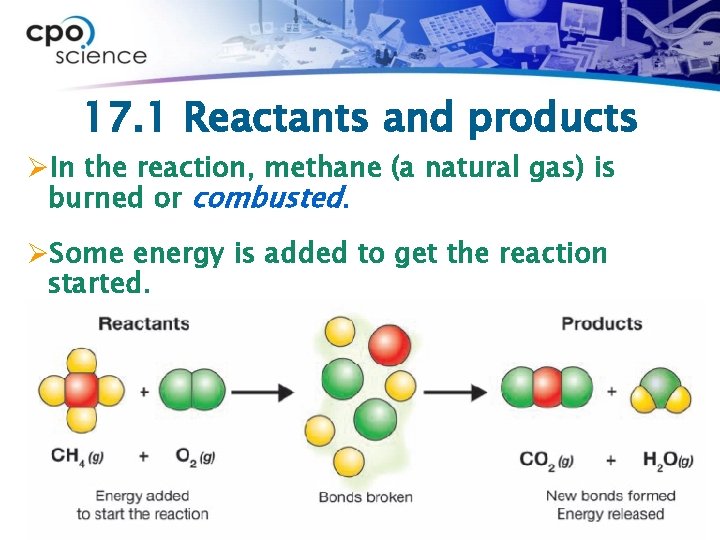
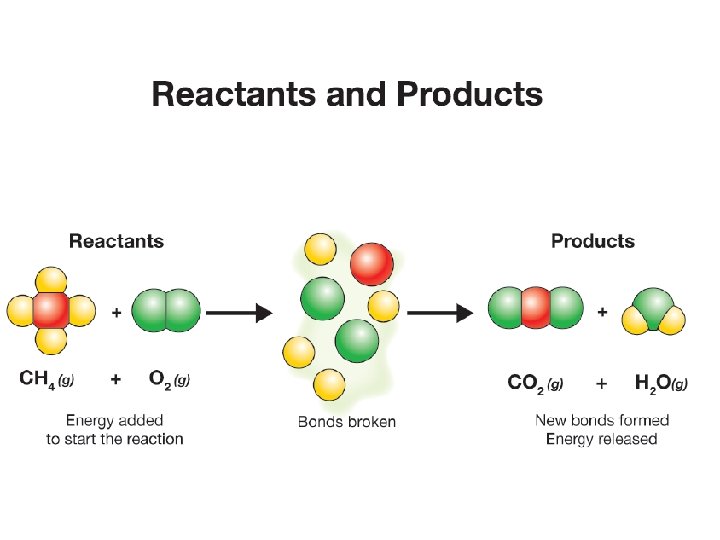
17. 1 Reactants and products ØIn the reaction, methane (a natural gas) is burned or combusted. ØSome energy is added to get the reaction started.


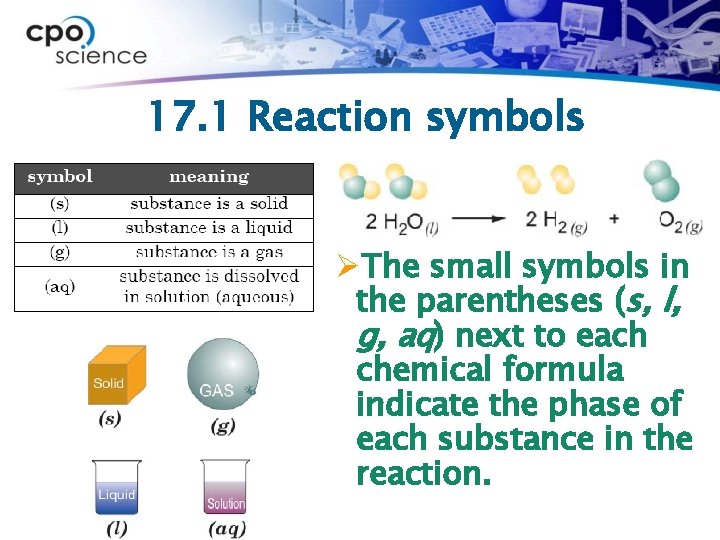
17. 1 Reaction symbols ØThe small symbols in the parentheses (s, l, g, aq) next to each chemical formula indicate the phase of each substance in the reaction.

Chapter Seventeen: Chemical Change Ø 17. 1 Chemical Reactions Ø 17. 2 Balancing Equations Ø 17. 3 Classifying Reactions

Chapter 17. 2 Learning Goals ØRelate a balanced chemical equation to the law of conservation of mass. ØDetermine the formula and molar masses of chemical compounds. ØWrite and balance chemical equations.

Investigation 17 B Conservation of Mass ØKey Question: How do scientists describe what happens in a chemical reaction?

17. 2 Balancing Equations ØAntoine Laurent Lavoisier, established an important principal based on his experiments with chemical reactions. ØHe stated that the total mass of the products of a reaction is equal to the total mass of the reactants. ØThe law of conservation of mass holds true for even a burning mass of wood.

17. 2 Balancing Equations ØThe combined mass of the burning wood and oxygen is converted into carbon dioxide and water.

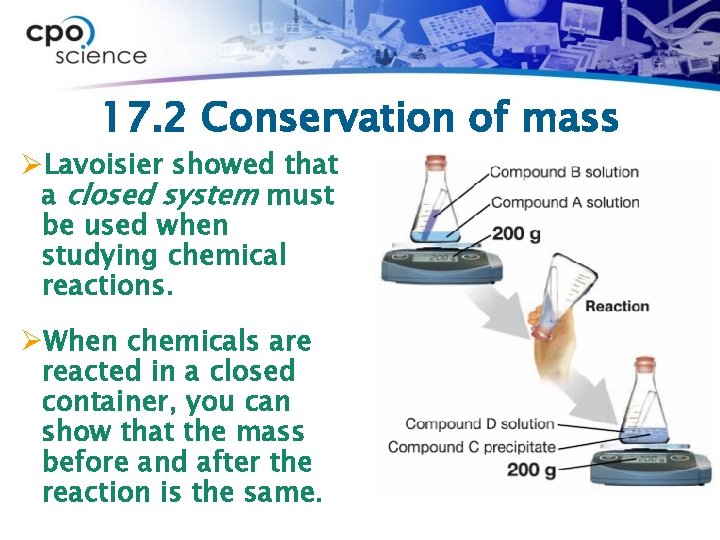
17. 2 Conservation of mass ØLavoisier showed that a closed system must be used when studying chemical reactions. ØWhen chemicals are reacted in a closed container, you can show that the mass before and after the reaction is the same.

17. 2 Formula mass ØThe sum of the atomic mass values of the atoms in a chemical formula is called the formula mass.

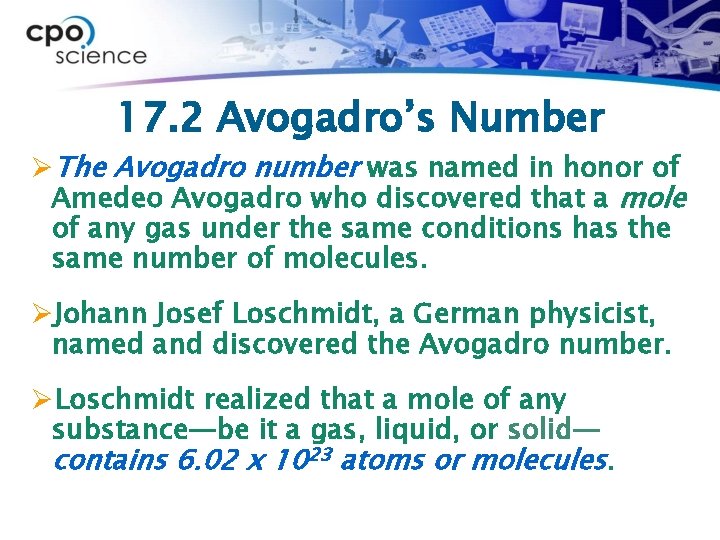
17. 2 Avogadro’s Number ØThe Avogadro number was named in honor of Amedeo Avogadro who discovered that a mole of any gas under the same conditions has the same number of molecules. ØJohann Josef Loschmidt, a German physicist, named and discovered the Avogadro number. ØLoschmidt realized that a mole of any substance—be it a gas, liquid, or solid— contains 6. 02 x 1023 atoms or molecules.

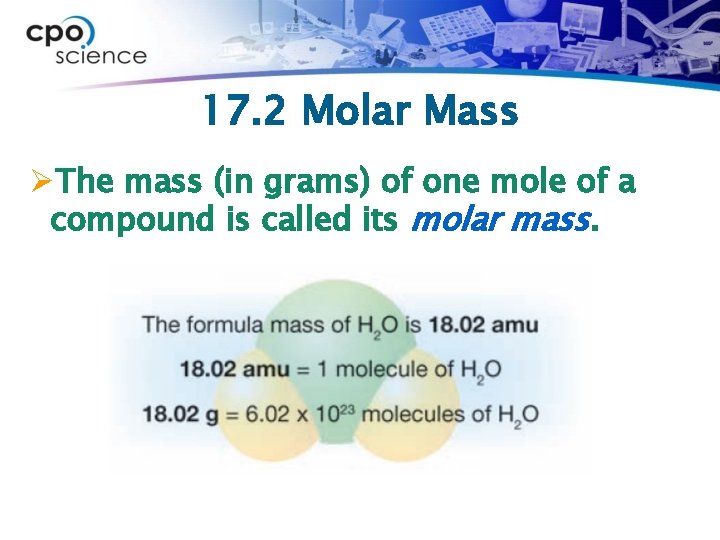
17. 2 Molar Mass ØThe mass (in grams) of one mole of a compound is called its molar mass.

Solving Problems What is the molar mass of one mole of Ca. CO 3? 1. Looking for: Ø … molar mass of Ca. CO 3 2. Given Ø … chemical formula 3. Relationships: Ø no. amu in formula = molar mass in grams

Solving Problems 4. Solution Ø Use periodic table and round values as needed. Formula mass Ca. C 03 = 100. 19 g 1 mole Ca. C 03 = 100. 19 g Ca. CO 3


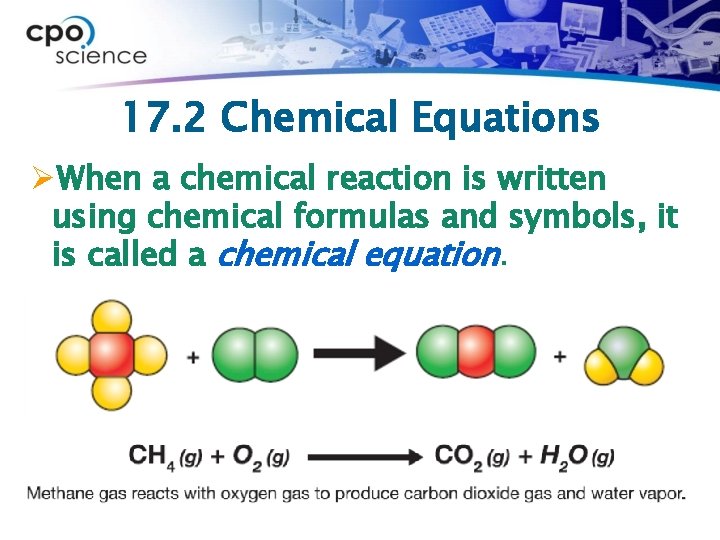
17. 2 Chemical Equations ØWhen a chemical reaction is written using chemical formulas and symbols, it is called a chemical equation.

17. 2 Chemical equations ØAn arrow is always included between reactants and products. ØIt means “to produce” or “to yield. ” to produce Reactants Products “Methane combines with oxygen gas to produce carbon dioxide gas and water vapor. ”

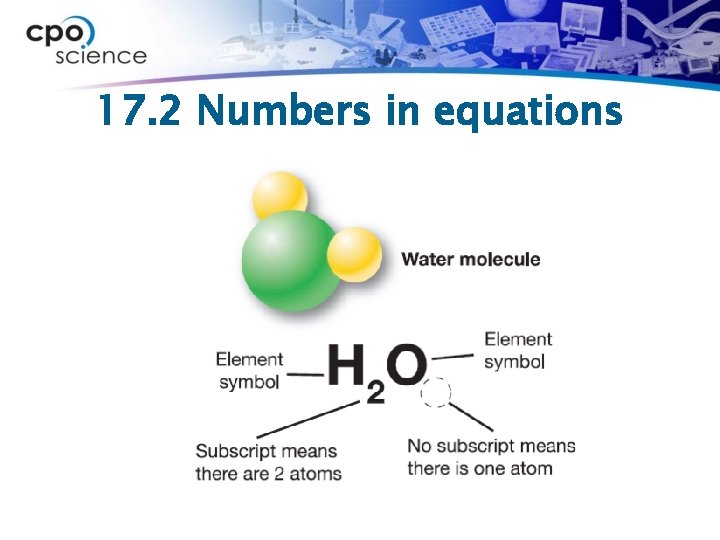
17. 2 Numbers in equations

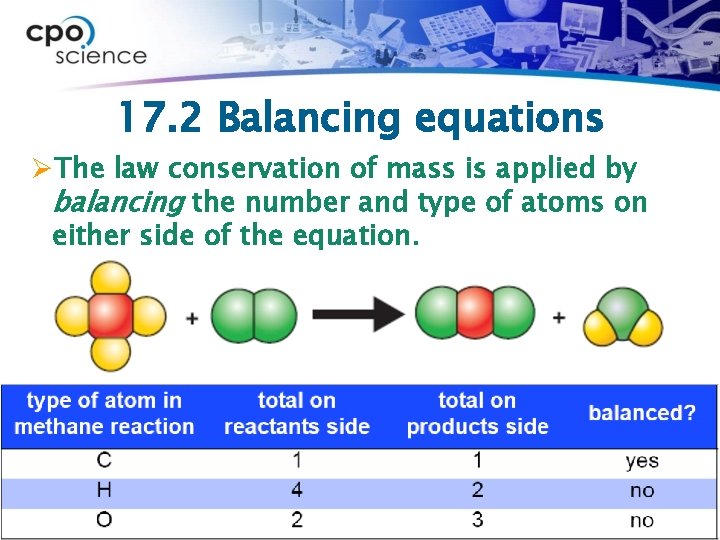
17. 2 Balancing equations ØThe law conservation of mass is applied by balancing the number and type of atoms on either side of the equation.

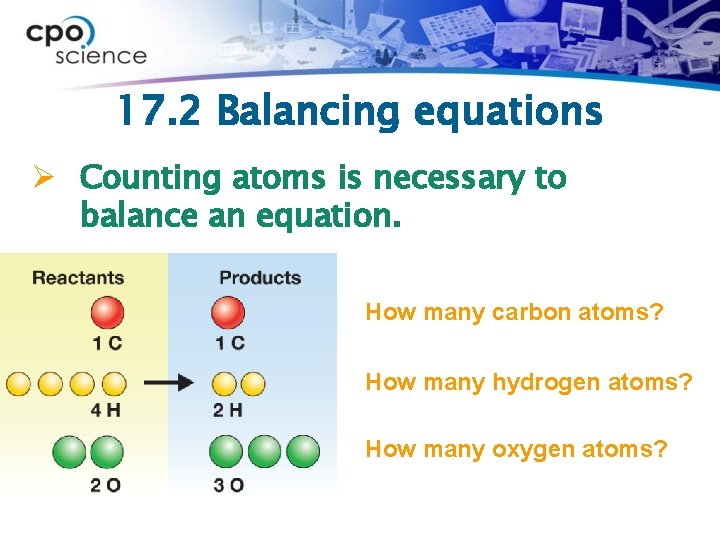
17. 2 Balancing equations Ø Counting atoms is necessary to balance an equation. How many carbon atoms? How many hydrogen atoms? How many oxygen atoms?

17. 2 Balancing chemical equations ØA balanced chemical equation has the same number of each type of atom on the product side and the reactant side. ØTo balance the equation, we add another water molecule to the product side and add another oxygen molecule to the reactant side. ØWe can practice balancing equations using CPO periodic table tiles and pencil and paper.

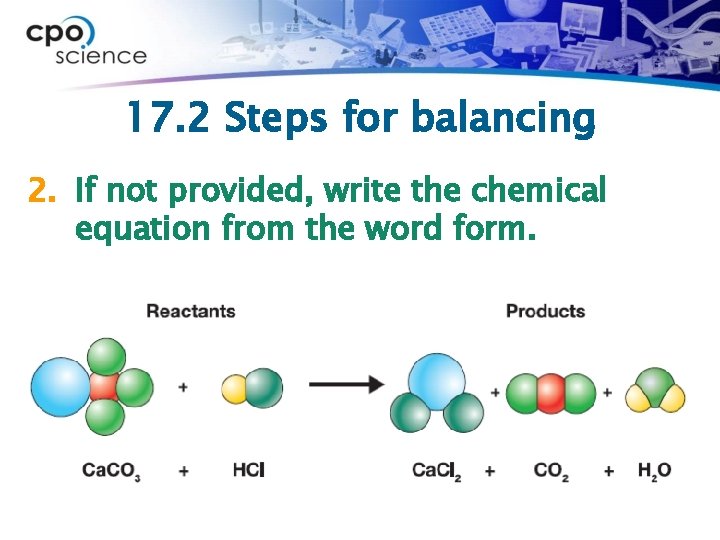
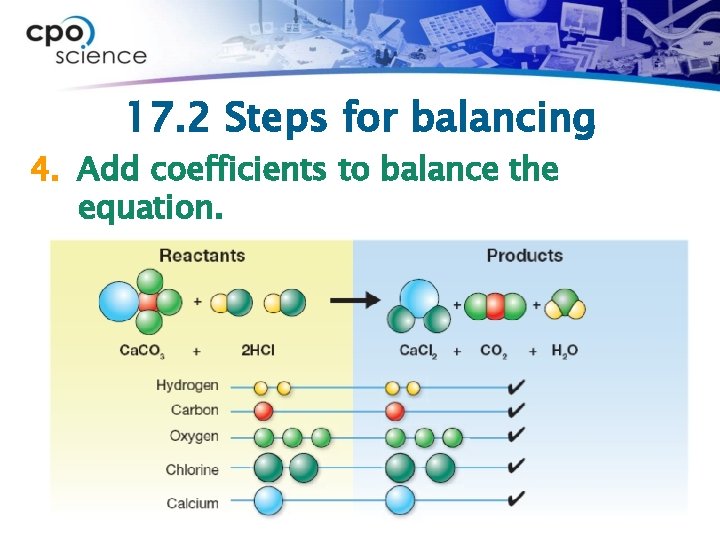
17. 2 Steps for balancing 1. If not provided, write the word form of the equation. Ø Calcium carbonate reacts with hydrochloric acid to produce calcium chloride, carbon dioxide and water.

17. 2 Steps for balancing 2. If not provided, write the chemical equation from the word form.

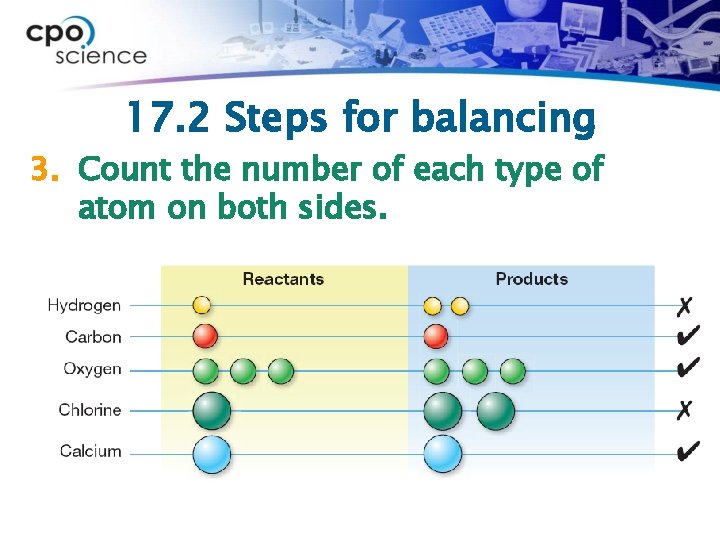
17. 2 Steps for balancing 3. Count the number of each type of atom on both sides.

17. 2 Steps for balancing 4. Add coefficients to balance the equation.


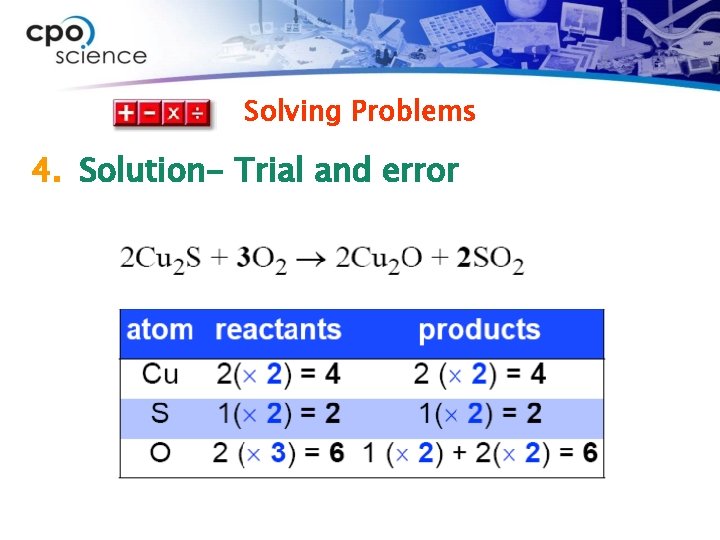
Solving Problems Ø In this reaction, chalcocite (a mineral) reacts with oxygen in the presence of heat. The products are a type of copper oxide and sulfur dioxide. Balance this equation: Cu 2 S + O 2 → Cu 2 O + SO 2

Solving Problems 1. Looking for: Ø …the coefficients for each molecule 2. Given Ø … chemical formulas which show types and no. of atoms

Solving Problems 3. Relationships Ø Coefficients can be added in front of any chemical formula in a chemical equation. Ø When a coefficient is added in front of a chemical formula, all atoms in that formula are multiplied by that number. Ø Use common denominators to help choose coefficients to try.

Solving Problems 4. Solution- Trial and error

Chapter Seventeen: Chemical Change Ø 17. 1 Chemical Reactions Ø 17. 2 Balancing Equations Ø 17. 3 Classifying Reactions

Chapter 17. 3 Learning Goals ØClassify reactions based on how atoms combine to create new substances. ØDiscuss applications of polymer science. ØStudy examples of combustion reactions.

Investigation 17 C Classifying Chemical Reactions ØKey Question: How can you predict the products of a chemical reaction?

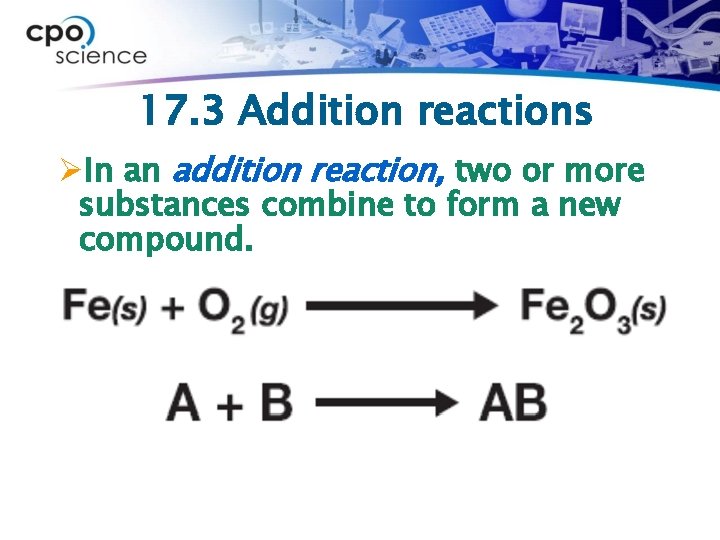
17. 3 Addition reactions ØThe process of creating large molecules from small ones is called polymerization.

17. 3 Addition reactions ØIn an addition reaction, two or more substances combine to form a new compound.

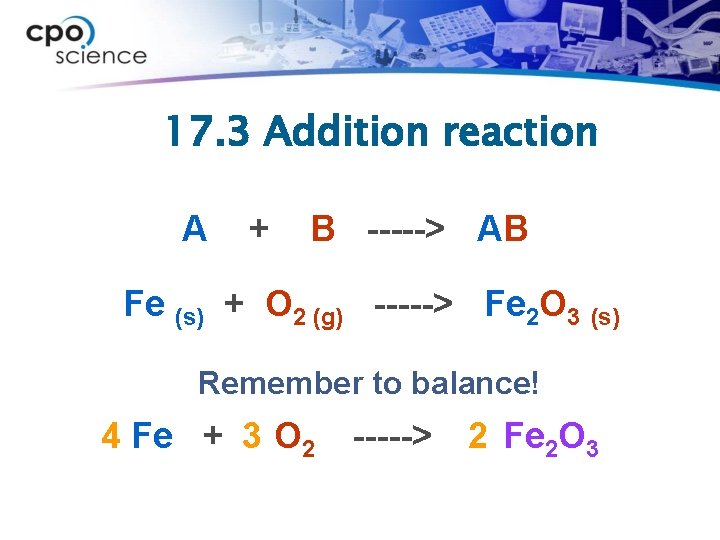
17. 3 Addition reaction A + B -----> AB Fe (s) + O 2 (g) -----> Fe 2 O 3 (s) Remember to balance! 4 Fe + 3 O 2 -----> 2 Fe 2 O 3

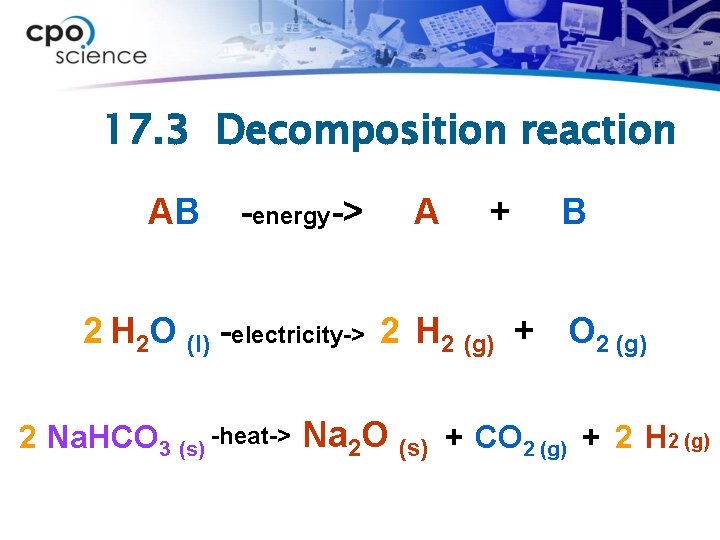
17. 3 Decomposition reactions ØA chemical reaction in which a single compound is broken down to produce two or more smaller compounds is called a decomposition reaction.

17. 3 Decomposition reaction AB -energy-> A + B 2 H 2 O (l) -electricity-> 2 H 2 (g) + O 2 (g) 2 Na. HCO 3 (s) -heat-> Na 2 O (s) + CO 2 (g) + 2 H 2 (g)


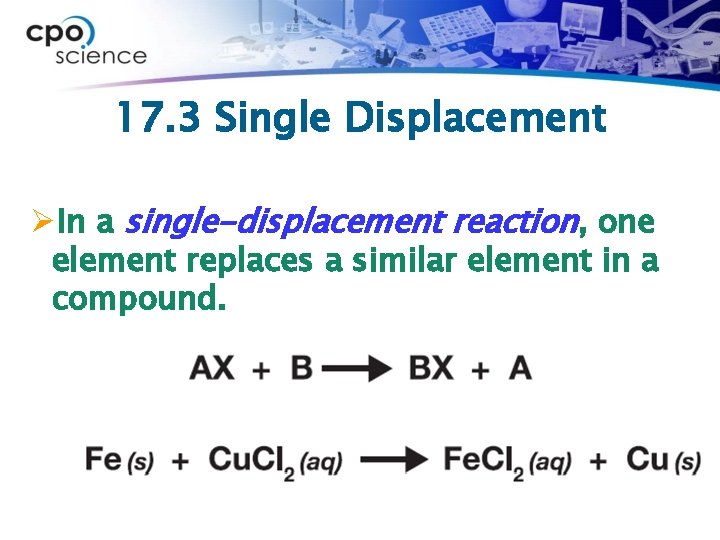
17. 3 Single Displacement ØIn a single-displacement reaction, one element replaces a similar element in a compound.

17. 3 Single Displacement A + XB Fe + Cu Cl 2 -----> + +

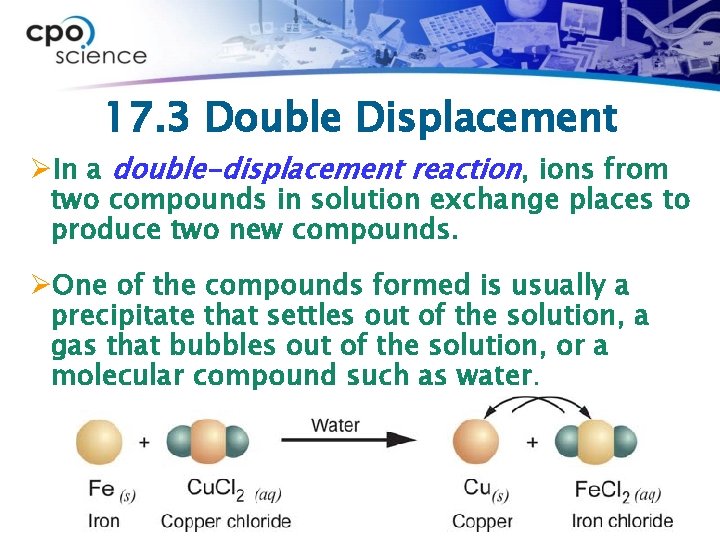
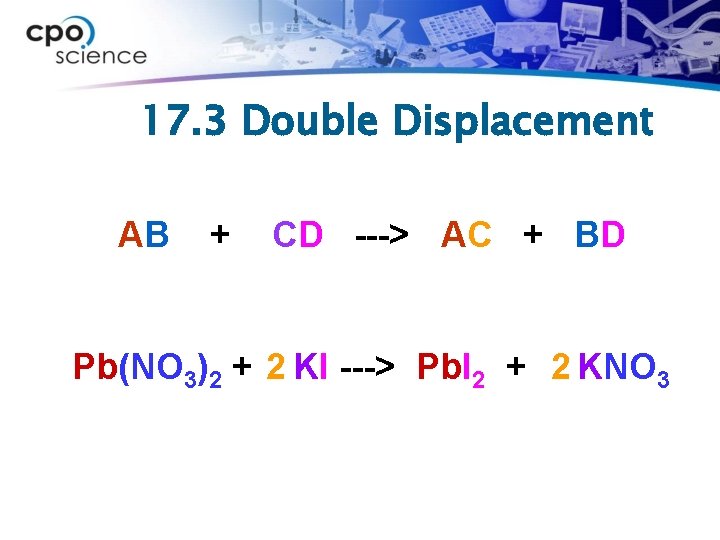
17. 3 Double Displacement ØIn a double-displacement reaction, ions from two compounds in solution exchange places to produce two new compounds. ØOne of the compounds formed is usually a precipitate that settles out of the solution, a gas that bubbles out of the solution, or a molecular compound such as water.

17. 3 Double Displacement AB + CD ---> AC + BD Pb(NO 3)2 + 2 KI ---> Pb. I 2 + 2 KNO 3

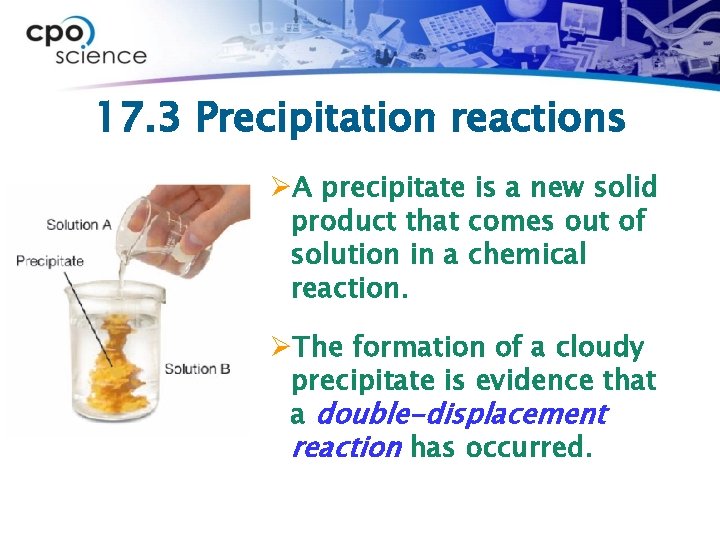
17. 3 Precipitation reactions ØA precipitate is a new solid product that comes out of solution in a chemical reaction. ØThe formation of a cloudy precipitate is evidence that a double-displacement reaction has occurred.

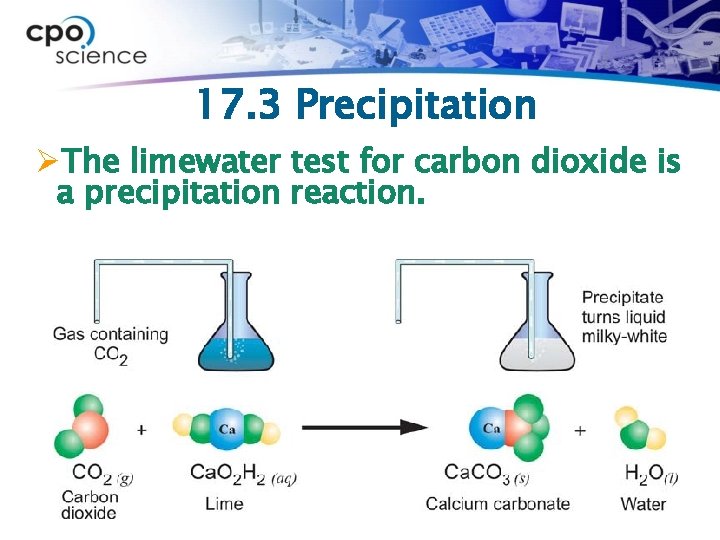
17. 3 Precipitation ØThe limewater test for carbon dioxide is a precipitation reaction.

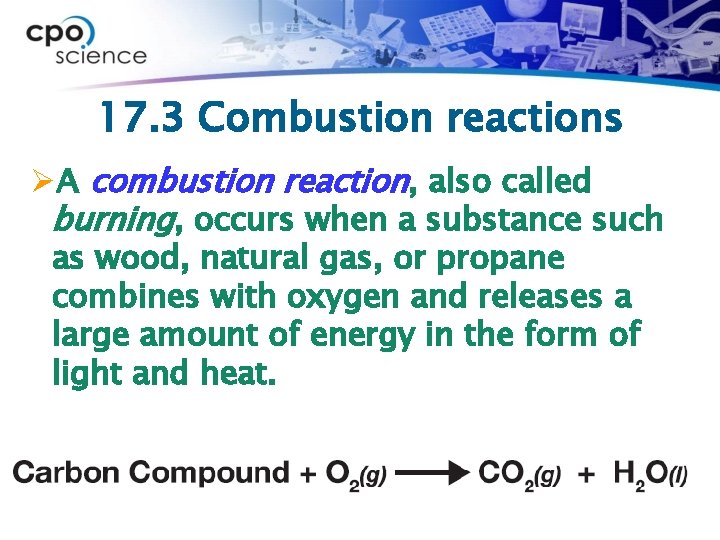
17. 3 Combustion reactions ØA combustion reaction, also called burning, occurs when a substance such as wood, natural gas, or propane combines with oxygen and releases a large amount of energy in the form of light and heat.

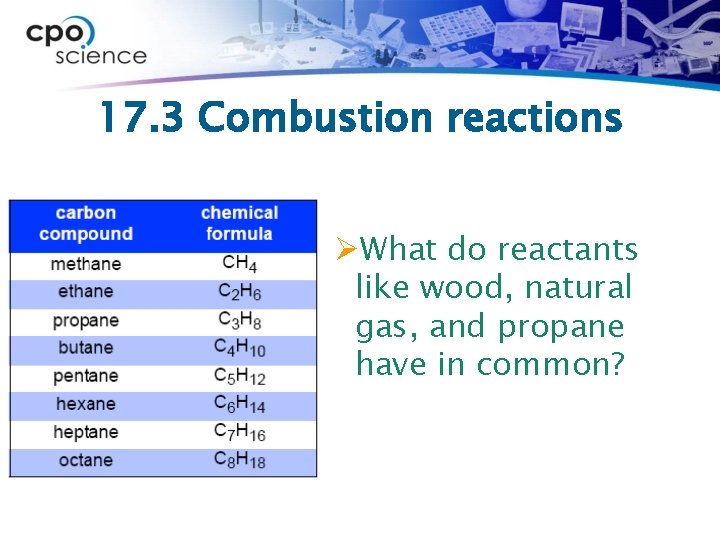
17. 3 Combustion reactions ØWhat do reactants like wood, natural gas, and propane have in common?

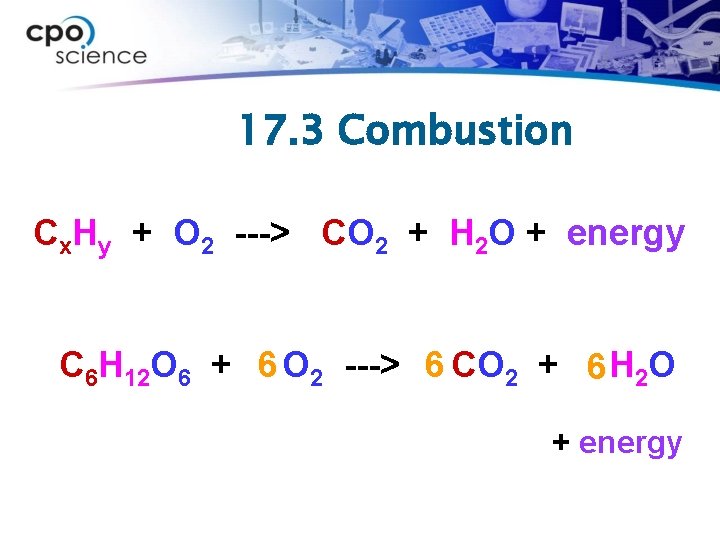
17. 3 Combustion Cx. Hy + O 2 ---> CO 2 + H 2 O + energy C 6 H 12 O 6 + 6 O 2 ---> 6 CO 2 + 6 H 2 O + energy


Hydrogen Powered Cars Ø Scientists and engineers from government agencies, universities, and all of the major automobile manufacturers are designing, building, and testing hydrogen fuel cell vehicles, also known as FCVs.
 Dance moms seventeen magazine
Dance moms seventeen magazine Seventeen symbols
Seventeen symbols Parfoy
Parfoy Seventeen table
Seventeen table Is mashing potatoes a physical or chemical change
Is mashing potatoes a physical or chemical change What is a phisical change
What is a phisical change Difference between chemical and physical property
Difference between chemical and physical property Physical change and chemical change
Physical change and chemical change Spare change physical versus chemical change
Spare change physical versus chemical change Whats a chemical change
Whats a chemical change Physical change
Physical change How does a physical change differ from a chemical change?
How does a physical change differ from a chemical change? Chopping wood physical or chemical
Chopping wood physical or chemical Empirical formula pogil
Empirical formula pogil Love formula in chemistry
Love formula in chemistry Are kc and kp equal
Are kc and kp equal Water vaporization equation
Water vaporization equation Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chemical reactions test answers
Chapter 9 chemical reactions test answers Tribromine octoxide formula
Tribromine octoxide formula Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 1 chemical changes
Section 1 chemical changes Is tearing paper a physical change
Is tearing paper a physical change Undesirable change in the physical chemical
Undesirable change in the physical chemical Baking cookies chemical or physical change
Baking cookies chemical or physical change Aluminum foil is cut in half chemical or physical change
Aluminum foil is cut in half chemical or physical change Cream being whipped physical or chemical
Cream being whipped physical or chemical First sign of puberty in males
First sign of puberty in males Physical property of blue color
Physical property of blue color Physical properties
Physical properties Chemical vs physical change particle diagram
Chemical vs physical change particle diagram Is separating sand from gravel a physical change
Is separating sand from gravel a physical change Grating cheese chemical or physical change
Grating cheese chemical or physical change Is rotting garbage a chemical change
Is rotting garbage a chemical change Observing chemical change worksheet
Observing chemical change worksheet Physical change of bread
Physical change of bread Grade 11 quantitative aspects of chemical change
Grade 11 quantitative aspects of chemical change Chemical change grade 10
Chemical change grade 10 Is dissolving salt in water chemical or physical change
Is dissolving salt in water chemical or physical change Is the stomach a physical or chemical change
Is the stomach a physical or chemical change Combustion chemical reaction definition
Combustion chemical reaction definition Zinc silver nitrate gives zinc nitrate silver
Zinc silver nitrate gives zinc nitrate silver How to balance an equation step by step
How to balance an equation step by step Inflating a balloon physical or chemical change
Inflating a balloon physical or chemical change Is a bike rusting a chemical change
Is a bike rusting a chemical change 5 indicators of a chemical change
5 indicators of a chemical change 5 indicators of chemical reaction
5 indicators of chemical reaction Whats the difference between chemical and physical change
Whats the difference between chemical and physical change Is it a physical or chemical change
Is it a physical or chemical change Is sawing wood a chemical change
Is sawing wood a chemical change Is separating sand from gravel a chemical change
Is separating sand from gravel a chemical change Chemical change signs
Chemical change signs Physcial change
Physcial change Mixing red and green marbles physical change
Mixing red and green marbles physical change Chemical change
Chemical change Is it a physical or chemical change
Is it a physical or chemical change Can a physical change be reversed
Can a physical change be reversed Is pancakes cooking a chemical or physical change
Is pancakes cooking a chemical or physical change School architecture change chemical
School architecture change chemical Chemical change of milk
Chemical change of milk