CHEM 100 Mira Costa College Fall 2016 IS










- Slides: 14

CHEM 100 Mira. Costa College Fall 2016

IS THIS COURSE FOR YOU? This course is designed for general education and allied health majors. If you are a science or premed major. This course is non-transferrable.

COURSE MATERIALS General, Organic and Biological Chemistry – Structures of Life: 4 th Edition Pearson MCC Custom Text – 2014 by Karen C. Timberlake. Laboratory Experiments for CHEM 100: Digital download from Blackboard Safety Goggles: Must have splashshielded vents (Z-87 rated) Scientific Calculator

IMPORTANT DATES: 9/2 - Last day to add full-term classes with instructor permission - Last day to drop full-term classes for a full refund and no record 9/5 - Campus Closed - Labor Day 11/11 - Campus Closed - Veterans Day 11/18 - Withdrawal (W) deadline for full-term classes 11/24 & 11/25 - Campus Closed - Thanksgiving 12/12 - Final Exam

HOW TO REACH ME? Email: awilliford@miracosta. edu Office hours: Every Mon 8: 45 – 10: 00 in room 7053 (HORT BLDG) Before class/after class If you email me, I will generally get back to you within 24 hours, but it could take me as long as two days.

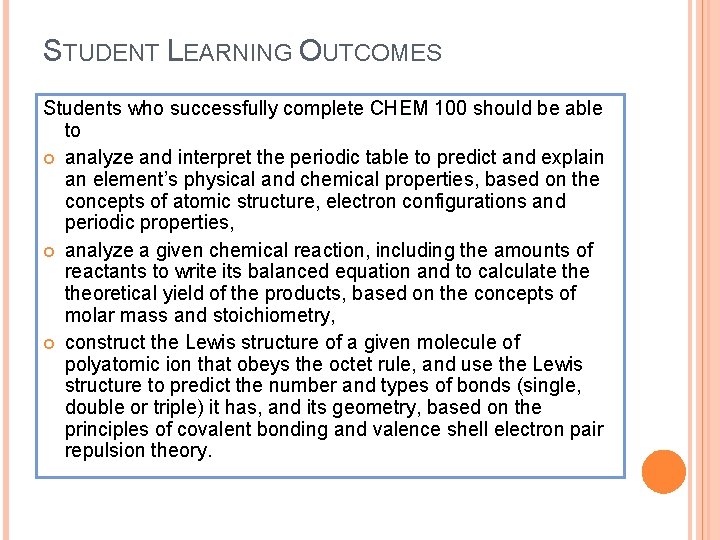
STUDENT LEARNING OUTCOMES Students who successfully complete CHEM 100 should be able to analyze and interpret the periodic table to predict and explain an element’s physical and chemical properties, based on the concepts of atomic structure, electron configurations and periodic properties, analyze a given chemical reaction, including the amounts of reactants to write its balanced equation and to calculate theoretical yield of the products, based on the concepts of molar mass and stoichiometry, construct the Lewis structure of a given molecule of polyatomic ion that obeys the octet rule, and use the Lewis structure to predict the number and types of bonds (single, double or triple) it has, and its geometry, based on the principles of covalent bonding and valence shell electron pair repulsion theory.

HOW TO BE SUCCESSFUL IN THIS COURSE Come to class Pay attention Do the assignments – ask me if you have problems you don’t understand Embrace the course – be happy like the hippo Along Came Polly Check Blackboard often

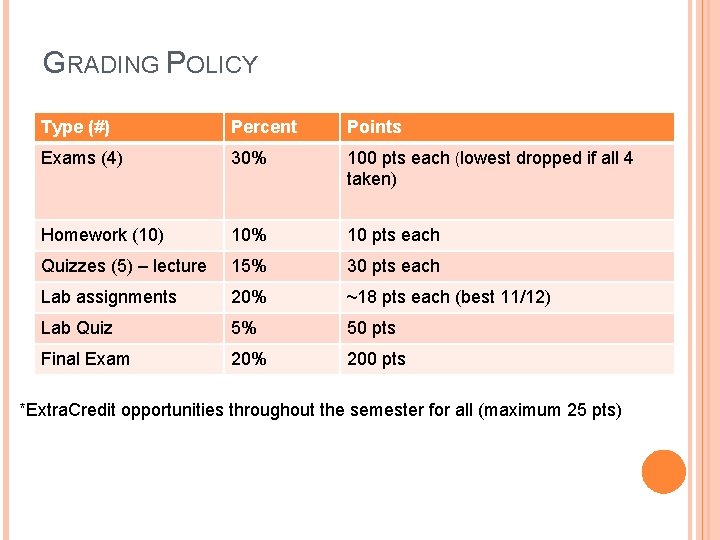
GRADING POLICY Type (#) Percent Points Exams (4) 30% 100 pts each (lowest dropped if all 4 taken) Homework (10) 10% 10 pts each Quizzes (5) – lecture 15% 30 pts each Lab assignments 20% ~18 pts each (best 11/12) Lab Quiz 5% 50 pts Final Exam 20% 200 pts *Extra. Credit opportunities throughout the semester for all (maximum 25 pts)

HOW IMPORTANT IS THE LAB? A passing lab grade of 70% is required to pass the course It is 25% of your overall grade The laboratory is YOUR chance to apply the concepts we are learning What to do: Come prepared Follow all safety rules Be curious Ask questions!

ACADEMIC HONESTY Plagiarism and Cheating WILL NOT BE TOLERATED This includes but is not limited to: � Direct copying of another student's assignment, test, or other required work; � Allowing or not preventing another student to look at your paper during a quiz or exam; � Looking at another student's paper during a quiz or exam; � Using notes, books, cameras, un-allowed calculators, cell phones, or other text-capable devices during a quiz or exam


BE RESPECTFUL Cell phones – PLACE ON SILENT � Please, do not answer your phone or send/receive text during lecture � Do not use as a calculator Laptop and tablet usage during class must be focused on our classwork and may be prohibited during certain in-class activities (exams/quizzes) Respect everyone’s learning experience


SYLLABUS QUIZ 1. Break out into teams 2. Name your team after a famous scientist 3. One sheet per team 4. Answer following questions – prizes for 1 st, 2 nd and 3 rd teams to finish with all answers correct. 5. Bring up your sheet when completed.
 100 100 100 100 100
100 100 100 100 100 Mira costa surf
Mira costa surf Account form balance sheet
Account form balance sheet Mira costa rugby
Mira costa rugby Mira costa counseling
Mira costa counseling Chempro 100i training
Chempro 100i training Free fall 2016
Free fall 2016 Contra costa college concurrent enrollment
Contra costa college concurrent enrollment 300 sayısının yüzde 30 u kaçtır
300 sayısının yüzde 30 u kaçtır 200+200+100+100
200+200+100+100 Box plot gcse
Box plot gcse Malloc lab 100/100
Malloc lab 100/100 Héroïne dans la guerre de 100 ans (100 years war).
Héroïne dans la guerre de 100 ans (100 years war). 100+100=200
100+100=200 Big data on bare metal
Big data on bare metal