Chapter 5 The Gaseous State Chemistry 1061 Principles










- Slides: 12

Chapter 5: The Gaseous State Chemistry 1061: Principles of Chemistry I Andy Aspaas, Instructor

Pressure • Pressure: force per unit area – Force exerted on a surface area by molecules in motion • Units: 1 atmosphere = 14. 7 psi 1 atmosphere = 760 mm Hg 1 atmosphere = 101, 325 Pascals 1 Pascal = 1 kg/m·s 2

Boyle’s law • Relates volume and pressure of a gas • PV = constant • Pi. Vi=Pf. Vf

Charles’s Law • Relates volume and temperature of a gas • V/T = constant • Vi / T i = Vf / Tf

Combined gas law • Combines Charles’s and Boyle’s laws to give one equation • (Pi. Vi)/Ti = (Pf. Vf)/Tf

Avogadro’s Law • Relates volume with amount of gas • Equal volumes of any two gases at the same temperature and pressure contain the same number of molecules • Volume of 1 mole of gas = Vm, molar gas volume • 22. 4 L/mol at STP (standard temp & pressure, 0 °C and 1 atm) • Nearly the same for any ideal gas!

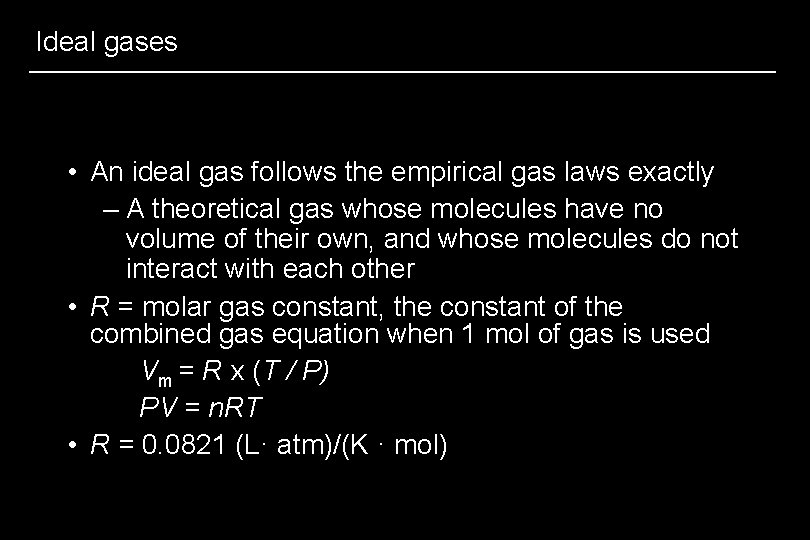
Ideal gases • An ideal gas follows the empirical gas laws exactly – A theoretical gas whose molecules have no volume of their own, and whose molecules do not interact with each other • R = molar gas constant, the constant of the combined gas equation when 1 mol of gas is used Vm = R x (T / P) PV = n. RT • R = 0. 0821 (L· atm)/(K · mol)

Using the ideal gas law • If moles gas can be calculated… – Moles mass density (if MW is known) – Moles MW (if mass is given) – Etc. • Stoiciometry in equations can give number of moles – Moles Liters by ideal gas law

Law of partial pressures • The sum of partial pressures (PA) of all of the different gases in a mixture is equal to the total pressure of the mixture • Related to mole fraction of a component of a mixture – Mole fraction: fraction of moles of a certain substance in a mixture of several substances – Mole fraction = (n. A/n) = (PA/P) • Vapor pressure of water, when collecting gases over water

Molecular speed and effusion • Average molecular speed, (u) is dependent on the temperature and Molar mass of the gas u = [(3 RT) / (Mm)]1/2 • Effusion: process in which a gas flows through a small hole in a container – Rate of effusion is proportional to 1/ (Mm)1/2

Real gases • Ideal gas equation does not hold up, especially at high pressures – Real volume is larger than the ideal gas law predicts, since molecules themselves take up space – Actual pressure is smaller than ideal gas law predicts, since intermolecular attractions weaken collisions against the walls of the vessel

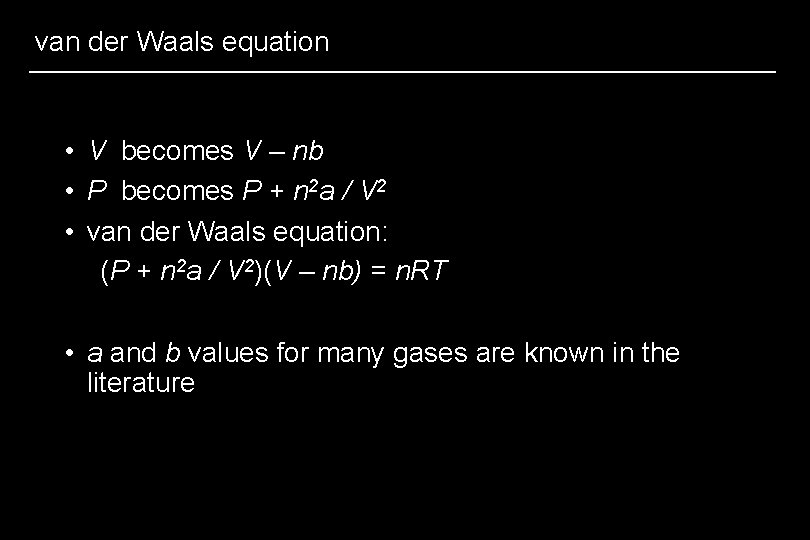
van der Waals equation • V becomes V – nb • P becomes P + n 2 a / V 2 • van der Waals equation: (P + n 2 a / V 2)(V – nb) = n. RT • a and b values for many gases are known in the literature