Chapter 5 1 CHEMICAL REACTIONS CHEMICAL REACTIONS CHANGE





- Slides: 10

Chapter 5. 1 CHEMICAL REACTIONS

CHEMICAL REACTIONS CHANGE SUBSTANCES � When sugar, water, and yeast are mixed with flour to make bread dough, a chemical reaction takes place � Yeast acts on sugar to form carbon dioxide and lactic acid � Different from sugar � Sugar is sweet, lactic acid is sour � Chemical reactions occur when substances undergoe chemical changes to form new substances

CHEMICAL REACTIONS CHANGE SUBSTANCES � Production of gas and change of color are signs of chemical reactions � In bread, the carbon dioxide gas produces expands the dough � As the dough bakes, old bonds break and new bonds form � Reactions involving starch and protein make food turn brown when heated (pg 149)

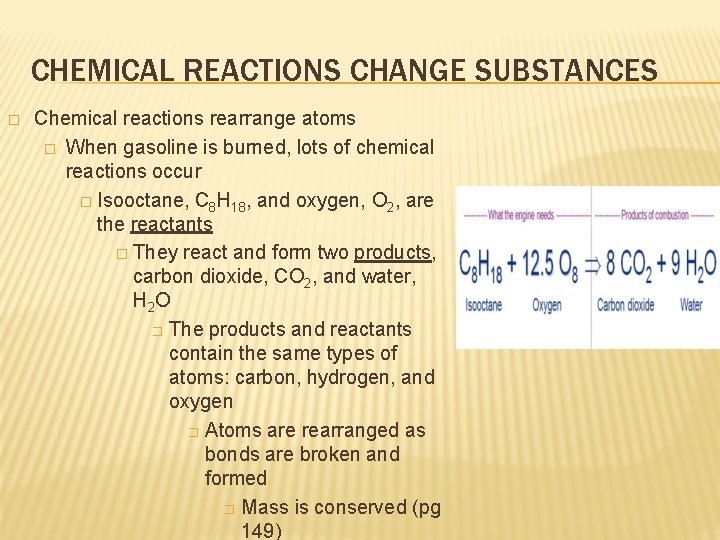
CHEMICAL REACTIONS CHANGE SUBSTANCES � Chemical reactions rearrange atoms � When gasoline is burned, lots of chemical reactions occur � Isooctane, C 8 H 18, and oxygen, O 2, are the reactants � They react and form two products, carbon dioxide, CO 2, and water, H 2 O � The products and reactants contain the same types of atoms: carbon, hydrogen, and oxygen � Atoms are rearranged as bonds are broken and formed � Mass is conserved (pg 149)

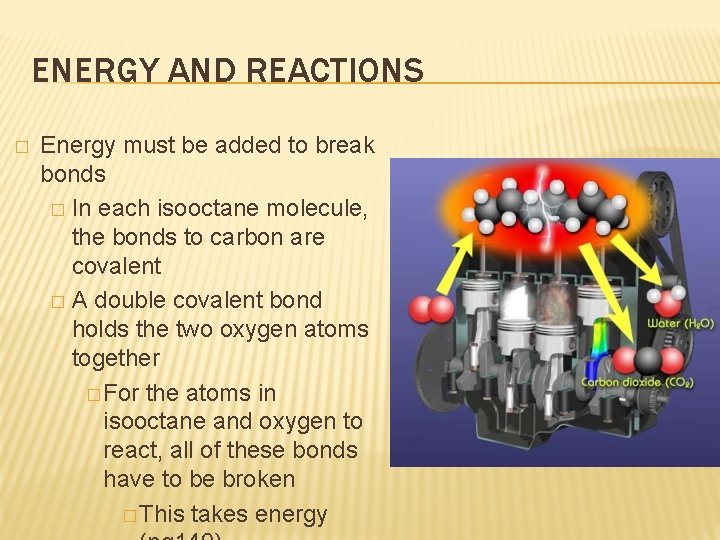
ENERGY AND REACTIONS � Energy must be added to break bonds � In each isooctane molecule, the bonds to carbon are covalent � A double covalent bond holds the two oxygen atoms together � For the atoms in isooctane and oxygen to react, all of these bonds have to be broken � This takes energy

ENERGY AND REACTIONS � Forming bonds releases energy � Once energy is added to start the isooctaneoxygen reaction, new bonds form to make the products � When new bonds form, energy is released � When gasoline burns, energy in the form of heat

ENERGY AND REACTIONS � Energy is conserved in chemical reactions � The energy released during the reaction is stored in the reactants � Stored energy is called chemical energy � Total energy before the reaction is equal to the total energy of the products (pg 151)

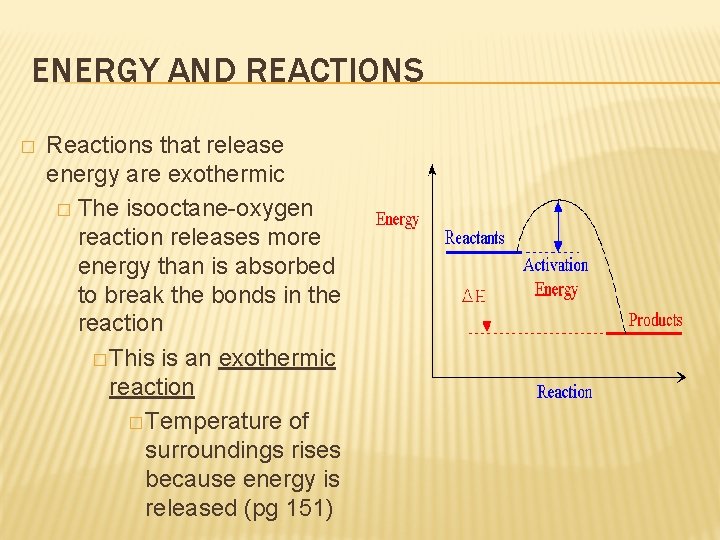
ENERGY AND REACTIONS � Reactions that release energy are exothermic � The isooctane-oxygen reaction releases more energy than is absorbed to break the bonds in the reaction � This is an exothermic reaction � Temperature of surroundings rises because energy is released (pg 151)

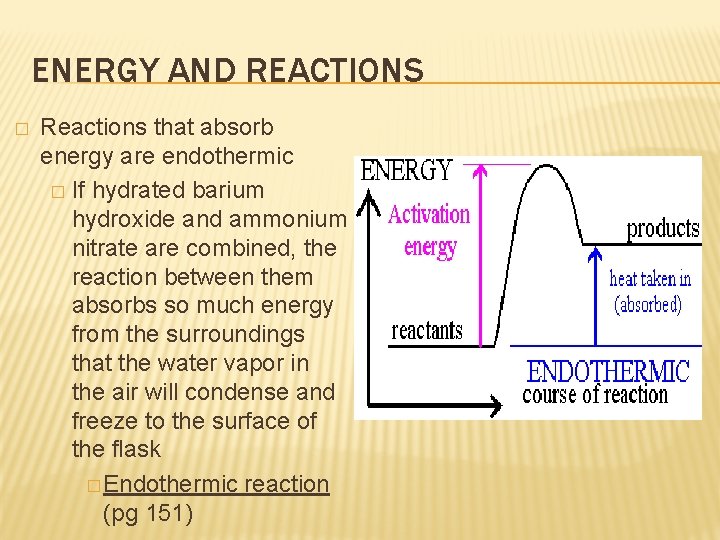
ENERGY AND REACTIONS � Reactions that absorb energy are endothermic � If hydrated barium hydroxide and ammonium nitrate are combined, the reaction between them absorbs so much energy from the surroundings that the water vapor in the air will condense and freeze to the surface of the flask � Endothermic reaction (pg 151)

ENERGY AND REACTIONS � Some endothermic reactions cannot get enough energy from the surroundings, so energy must be added to get the reaction started � Photosynthesis is an endothermic reaction � Plants use light energy convert carbon dioxide and water to glucose and oxygen (pg 152)
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Are kc and kp equal
Are kc and kp equal Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key Chapter 9 chemical reactions
Chapter 9 chemical reactions Section 1 chemical changes
Section 1 chemical changes Examples for physical change
Examples for physical change What are chemical changes
What are chemical changes Differences between chemical and physical changes
Differences between chemical and physical changes Examples of physical vs chemical changes
Examples of physical vs chemical changes



















